Abstract
PURPOSE
External-beam accelerated partial breast irradiation (APBI) is an increasingly popular technique following treatment of patients for early-stage breast cancer with conventional breast-conserving therapy. Here we present 5-year results of a prospective trial.
METHODS AND MATERIALS
From 10/2003 through 11/2005, 98 evaluable patients with Stage I breast cancer were enrolled on the first dose-step (32 Gy delivered in 8 twice-daily fractions) of a prospective, multi-institutional, dose-escalation clinical trial of three-dimensional conformal external-beam APBI (3D-APBI).
The median age was 61 years; the median tumor size was 0.8 cm; 89% of tumors were estrogen receptor positive; 10% had a triple-negative phenotype; and 1% had a HER-2-positive subtype. Median follow-up was 71 months (range, 2–88 months; interquartile range 64–75 months).
RESULTS
Five patients developed IBTR, for a 5-year actuarial IBTR rate of 5% (95% confidence interval, 1–10%). Three of these occurred in patients with triple-negative disease and 2 in non-triple-negative patients, for 5-year actuarial IBTR rates of 33% (0–57%) and 2% (0–6%; p<0.0001), respectively. On multivariate analysis, triple-negative phenotype was the only predictor of IBTR, with borderline statistical significance after adjusting for tumor grade (p=0.0537).
CONCLUSIONS
Overall outcomes were excellent, particularly for patients with estrogen receptor positive disease. Patients in this study with triple-negative breast cancer had a significantly higher IBTR rate than patients with other receptor phenotypes when treated with 3D-APBI. Larger, prospective 3D-APBI clinical trials should continue evaluating the effect of hormone receptor phenotype on IBTR rates.
Keywords: 1. Breast cancer, 2. Triple-negative, 3. 3D conformal, 4. Prospective trial, 5. PBI
INTRODUCTION
Accelerated partial-breast irradiation (APBI) is increasingly used to treat patients with early-stage cancer who desire breast-conserving therapy (BCT) (1). The decreased volume of normal tissue irradiated, increased convenience for the patient with a shortened course of treatment, improved integration with chemotherapy, and possible decreases in treatment cost, make this approach appealing. However, there are few results thus far from prospective(2, 3) or randomized trials comparing APBI to whole-breast irradiation (WBI) (4, 5), and little evidence to guide clinicians regarding the suitability of patient subgroups for such treatment. Consensus guidelines for the suitability of APBI for select patients have been proposed, based on expert opinion derived from limited retrospective and prospective studies(6).
Several studies have found that “triple negative” (TN) tumors that do not express estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (Her-2) have higher risks of locoregional recurrence following BCT using whole-breast irradiation (WBI) than tumors expressing ER(7). For example, in one study, patients with TN tumors had a 5-year IBTR rate of 7%, as compared to 1–1.5% for tumors expressing ER. Some WBI studies have shown other results, including one that found similar 5-year IBTR rates for TN and non-TN tumors (17% for both groups) but the rates for non-TN patients were substantially higher than in other WBI studies(8). There are few studies evaluating the efficacy of APBI by receptor phenotype. In one study of patients treated using balloon brachytherapy, ER negativity was the only variable significantly associated with IBTR, with an odds ratio of 4.01(9). Two recent studies did not find that the TN phenotype was associated with a higher rate of IBTR after treatment exclusively or predominantly with brachytherapy APBI(10, 11). However, this subject has not been adequately studied, particularly for patients treated with three-dimensional conformal external-beam APBI (3D-APBI).
We investigated the outcome of patients treated in the first cohort of a prospective, dose-escalation, multi-institutional, clinical trial using 3D-APBI(12), whose median follow-up exceeds 5 years, assessing the prognostic importance of various clinical characteristics, including receptor phenotype.
METHODS AND MATERIALS
Protocol Goals, Registration, and Consent
This prospective, phase I, dose-escalation trial was approved by the relevant institutional review boards and opened to patient entry in September 2003 (Dana-Farber/Harvard Cancer Center protocol 03-179; registry number NCT00694577, clinicaltrials.gov). Its aims were to evaluate the technical feasibility, potential toxicities, and optimal dose for 3D-CRT APBI in select female breast cancer patients. The first dose step of 32 Gy in 8 fractions was based on previous trials(13) using interstitial brachytherapy to similar doses and achieving adequate IBTR rates. (Additional dose steps were 36 Gy in 9 fractions and 40 Gy in 10 fractions, with median follow-up times of 43 and 22 months, respectively; results will be reported as data mature.) Patients gave written informed consent in accordance with institutional and federal requirements. Patients were centrally registered after providing consent and could withdraw at any time. Stopping rules for the trial were not met and included, within 1 year of protocol registration, the occurrence of histological fat necrosis or other grade 4 skin or grade 4 subcutaneous toxicity, or the occurrence of an ipsilateral breast cancer recurrence or death related to treatment of 9% of enrolled patients.
Patient Selection
From October 2003 through November 2005, 100 patients with Stage I (AJCC 6th edition) breast cancer were enrolled on the first dose-step. Eligibility criteria included: age 18 years or older; Eastern Cooperative Oncology Group performance status of 0; invasive ductal carcinoma (including tubular, mucinous, and medullary variants); radiologic and/or pathologic tumor size 2 cm or smaller; uninvolved lymph nodes; microscopic tumor margins 2 mm or greater, or no tumor in a re-excision specimen or final shaved margin specimens. The protocol was revised in late 2004 to allow omission of sentinel lymph node biopsy or axillary lymph node dissection for patients above 70 years of age with ER-positive, T1, invasive ductal carcinoma, if the patient was to take hormonal therapy(14). Pretreatment evaluation with magnetic resonance imaging was optional. Placement of marking clips at the time of tumor excision was encouraged but not mandatory. Receptor status was determined by immunohistochemistry for ER, PR and Her-2. Generally, if the Her-2 result was equivocal, fluorescent in-situ hybridization results provided final determination of Her-2 status. Exclusion criteria included: presence of lymphovascular invasion or blood vessel invasion; presence of an extensive intraductal component; invasive lobular carcinoma or mixed ductal-lobular histology; testing showing a mutation known to predispose to breast cancer development, including BRCA1 or BRCA2; prior cosmetic or reconstructive breast surgery; psychiatric illness preventing the patient from giving informed consent; medical conditions such as uncontrolled infection (including HIV), uncontrolled diabetes mellitus, or connective tissue diseases which, in the opinion of the treating physician, would make this protocol unreasonably hazardous; pregnancy; or having a currently active second malignancy other than non-melanoma skin cancers. One patient elected not to receive treatment, and another withdrew her consent for inclusion in the study. Therefore, 98 patients are included in this analysis; 77 received treatment at the Massachusetts General Hospital, 16 at Beth Israel Deaconess Medical Center, and 5 at Boston Medical Center.
Treatment
Radiotherapy was required to begin within 4 to 12 weeks from the definitive breast surgery or, if chemotherapy was given first, within 2 to 6 weeks of the completion of chemotherapy. Chemotherapy could not be administered concurrently with APBI.
All patients underwent simulation on a dedicated computed tomography simulator after agreeing to enter the protocol. Patients were removed from the study if the radiation oncologist could not adequately delineate the excision cavity. Formal criteria for determining the extent of the excision cavity were not used. The planning target volume (PTV) was defined by expanding the outlined excision cavity by 1.5 to 2 cm, which was then edited so that it came no closer than 5 mm to the skin surface and was no deeper than the anterior chest wall or pectoralis muscle. Additional margin (typically 0.7 cm) was added to create the treatment field borders to account for penumbra.
Patients were treated to a dose of 32 Gy using 4 Gy fractions given twice daily, with at least 6 hours between fractions, over consecutive days so as to complete treatment within 1 week. Treatment could be started on any day of the week.
Treatment could be administered using any combination of photon beams of energy 4 MV or higher, with or without the addition of electrons of any energy, provided the dosimetric requirements of adequately treating the PTV and homogeneity were met. (Examples include mini-tangents plus en-face electrons, mini-tangents plus en-face photons, or wedge-pair photons plus en-face electrons or photons.) Intensity-modulated radiotherapy and proton beam radiation could also be used. A minimum of 3 beams was required, except for proton treatment. Noncoplanar plans were also allowed. Details of proton techniques have been previously described(15).
Dose was prescribed to the PTV. At least 95% of the PTV was to receive 100% of the prescribed dose. Maximal dose within the PTV was to be no greater than 115% of the prescribed dose. All doses were calculated using inhomogeneity corrections. Investigators were encouraged to reduce dose to critical structures as far as reasonably achievable. Plans were approved before the start of treatment by A.G.T., S.N.P. (for patients treated at Massachusetts General Hospital or Boston Medical Center), or A.R. (for patients treated at Beth Israel Deaconess Medical Center). Quality assurance or immobilization techniques beyond what was normally practiced at the participating institutions were not dictated by the protocol.
Systemic therapy was prescribed at the discretion of the treating physicians. Six patients (6%) received chemotherapy (doxorubicin and cyclophosphamide, 2 also with taxol), with or without hormone therapy, generally before APBI. Seventy patients (71%) received tamoxifen or an aromatase inhibitor. Hormone therapy was generally started after completion of radiotherapy.
Follow-Up
Patients were to be seen by the treating radiation oncologist 3 to 8 weeks after the completion of radiation, then every 6 months for the first 5 years, and then on an annual basis. Annual bilateral mammography was mandatory; although the majority of patients underwent imaging of the treated breast every 6 months with mammography or MRI (at the discretion of the treating physician).
Evaluation of Recurrences
For patients with an IBTR, we reviewed the operative reports, pathology reports, and imaging of the primary and recurrent tumor with an attending breast radiologist (H.D.). Measurement of distance between index and recurrent lesions was made with diagnostic mammograms and supplemented by MRI if available. An attending breast pathologist reviewed the histology and receptor immunohistochemistry of the primary and recurrent tumors (E.B.).
Statistical Considerations
The primary study endpoint was IBTR. Study variables included age, T-stage, tumor grade, ER status, TN breast cancer, use of systemic therapy, hormone therapy, chemotherapy and RT modality. Time until IBTR was censored on lost follow-up and on death. Local control rates at 60 months were reported using Kaplan-Meier estimates. Groups were compared using the log-rank test. Stratified Cox regression was used for multivariable analysis. A p-value of <0.05 was considered statistically significant.
RESULTS
The median follow-up for all patients was 71 months (range, 2–88 months; interquartile range 64–75 months).
Characteristics
Pretreatment characteristics for all patients are shown in Table I. The median patient age was 61 years. The median tumor size was 0.8 cm. Ninety of the patients' tumors (92%) were invasive ductal carcinomas, and 8 (8%) had other histology. Ten patients (10%) had no sentinel lymph nodes removed, 79 (81%) had 1 to 3 nodes removed, and 9 (9%) had 4 or more nodes removed. Ten patients (10%) had a pre-treatment MRI. Eighty-seven patients (89%) had ER-positive tumors, of whom 70 received hormonal therapy (80%); 11 patients (11%) had ER-negative tumors; 10 patients (10%) had TN tumors. Five of the 10 patients (50%) with TN tumors and one of 88 patients (1%) with non-TN tumors received chemotherapy. Characteristics by TN tumor status are shown in Table II.
Table I.
Patient, tumor and IBTR characteristics.
| Characteristic | Total (No.) | Total (%) | IBTR (No.) | 5-year IBTR rate (%) | P-value |
|---|---|---|---|---|---|
| All patients | 98 | 100% | 5 | 5% | - |
| Age: | |||||
| 40–49 | 15 | 15% | 2 | 14% | 0.4165 |
| 50–59 | 30 | 31% | 1 | 3% | |
| 60–69 | 32 | 33% | 1 | 3% | |
| >70 | 21 | 21% | 1 | 5% | |
| T stage: | |||||
| T1a | 21 | 21% | 0 | 0% | 0.1203 |
| T1b | 53 | 54% | 2 | 4% | |
| T1c | 24 | 24% | 3 | 13% | |
| Grade: | |||||
| 1 | 46 | 47% | 0 | 0% | 0.0205 |
| 2 | 42 | 43% | 3 | 7% | |
| 3 | 10 | 10% | 2 | 22% | |
| Estrogen Receptor Status: | |||||
| Positive | 87 | 89% | 2 | 2% | 0.0002 |
| Negative | 11 | 11% | 3 | 29% | |
| Triple-negative: | |||||
| Yes | 10 | 10% | 3 | 33% | <0.0001 |
| No | 88 | 90% | 2 | 2% | |
| Systemic therapy: | |||||
| Yes | 75 | 77% | 5 | 7% | 0.2321 |
| No | 23 | 23% | 0 | 0% | |
| Hormonal therapy: | |||||
| Yes | 70 | 71% | 2 | 3% | 0.0860 |
| No | 27 | 28% | 3 | 12% | |
| Chemotherapy: | |||||
| Yes | 6 | 6% | 3 | 50% | <0.0001 |
| No | 92 | 94% | 2 | 2% | |
| RT Modality: | |||||
| Photon & electrons | 60 | 61% | 3 | 5% | 0.3543 |
| Photon | 19 | 19% | 0 | 0% | |
| Proton | 19 | 19% | 2 | 11% | |
Table II.
Characteristics by TN tumor status.
| Characteristic | Total No. | TN No. (%) | Non-TN No. (%) | P-value |
|---|---|---|---|---|
| All patients | 98 | 10 | 88 | - |
| Age | ||||
| <50 | 15 | 3 (20%) | 12 (80%) | 0.1732 |
| ≥50 | 83 | 7 (8%) | 76 (92%) | |
| T stage | ||||
| T1a | 21 | 1 (5%) | 20 (95%) | 0.0223 |
| T1b | 53 | 3 (6%) | 50 (94%) | |
| T1c | 24 | 6 (25%) | 18 (75%) | |
| Grade | ||||
| 1 | 46 | 0 (0%) | 46 (100%) | <0.0001* |
| 2 | 42 | 4 (10%) | 38 (90%) | |
| 3 | 10 | 6 (60%) | 4 (40%) | |
Kendall's Tau = 0.43
IBTR
Five patients developed an IBTR at a median of 48 months after APBI (range, 30–55 months). All 5 patients were alive and well at last follow-up 22–44 months after salvage mastectomy. There were no regional nodal failures. One patient developed distant metastases with continued local control.
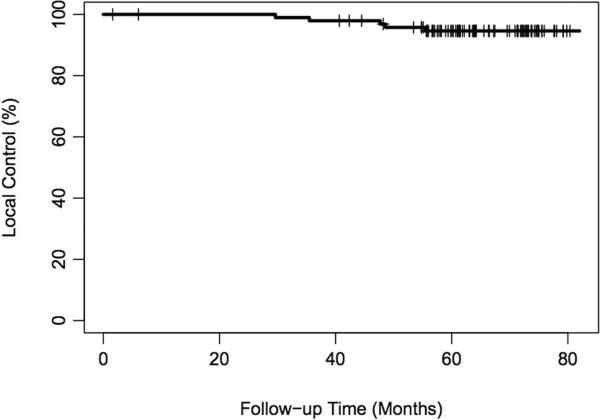
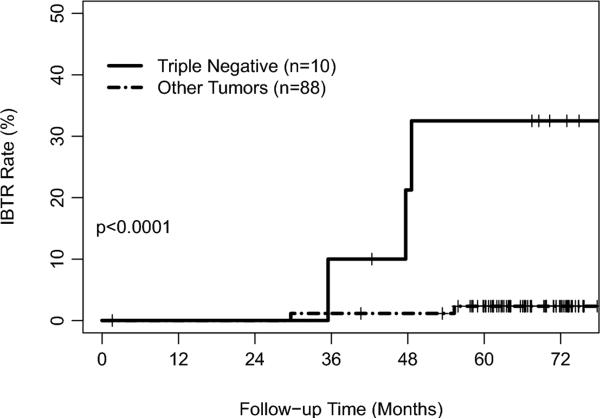
The 5-year actuarial IBTR rate was 5% (95% confidence interval, 1–10%) (Figure I). All patients suffering IBTR had received systemic treatment. On univariate analysis, TN tumor status, ER status and higher tumor grade were significant variables for IBTR (Table I). Three of 10 patients with TN tumors had IBTR, for a 5-year actuarial LR rate of 33% (0–57%). Two of 88 patients with non-TN tumors had IBTR, for a 5-year actuarial LR rate of 2% (0–6%; p<0.0001) [Figure II].
Figure I.
Overall rate of local control.
Figure II.
Ipsilateral breast tumor recurrence rate by triple-negative status.
Multivariable analysis was performed stratifying first for tumor grade and then TN status, as these were closely correlated. After stratification for tumor grade, TN status was the only significant predictor of IBTR, with a hazard ratio of 15.4 (2.6–92.9; p=0.0537). The effect of tumor grade on risk of IBTR was not significant after stratification for TN status (p=0.35).
An analysis of the effect of hormonal therapy on ER-positive patients and of chemotherapy on TN patients was performed. Two of 70 (3%) patients with ER expressing tumors treated with hormonal therapy developed an IBTR, compared to none among the 16 patients (0%) who did not receive hormonal therapy (p=0.503). Of the 10 TN patients, 3 of 5 (60%) receiving chemotherapy developed an IBTR; none of the 5 who did not receive it developed an IBTR (p=0.069).
We also examined the possible effect of the surgery-to-radiotherapy interval on the risk of IBTR. Two of the 3 TN patients with IBTR had chemotherapy prior to RT; RT commenced on days 62, 105, and 174 after lumpectomy (mean, 114 days). The 7 TN patients with local control had RT begin on days 39, 41, 48, 59, 60, 69 and 77 after lumpectomy (mean, 56 days). Thus, none of the 5 patients with TN tumors developed IBTR when RT was initiated within 60 days of lumpectomy, compared to 3 of 5 (60%) who started RT more than 60 days after lumpectomy.
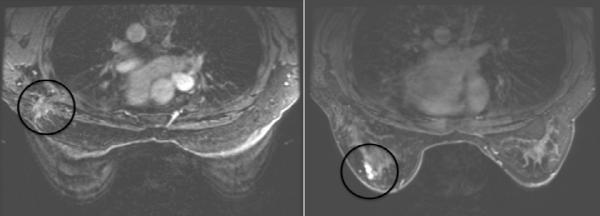
Characteristics of the 5 patients with IBTR are shown in Table III. None of the patients with IBTR had pre-treatment MRI. All IBTRs were located more than 3 cm from the site of the primary tumor (Figure III). All of the IBTRs had the same histology and receptor status as the initial tumors. In two TN cases, the grade changed (from grade 2 to grade 3).
Table III.
Characteristics of Patients with IBTR.
| Case | Age | Histo- logy |
Size (cm) |
Grade | Primary receptor status |
Hormonal therapy |
Chemo- therapy |
Interval to recurrence (months) |
Method of recurrence detection |
Treatment of recurrence |
Size of recurrent tumor |
Grade | Recurrence receptor status |
Disease status |
Interval since salvage (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | IDC | 1.8 | 3 | ER−, PR−, HER2− | N | Y | 48 | Mammo | M & ALND | 0.7 | ND | ER−, PR−, HER2− | ADF | 23 |
| 2 | 74 | IDC | 1 | 2 | ER+, PR+, HER2− | Y | N | 55 | Mammo | M & ALND | ND | ND | ER+, PR+, HER2− | ADF | 23 |
| 3 | 49 | IDC | 1.2 | 2 | ER−, PR−, HER2− | N | Y | 36 | MRI | Simple M | 1.2 | 3 | ER−, PR−, HER2− | ADF | 31 |
| 4 | 45 | IDC | 1 | 3 | ER−, PR−, HER2− | N | Y | 50 | MRI | M & ALND | 1.4 | 3 | ER−, PR−, HER2− | ADF | 22 |
| 5 | 62 | IDC | 1.3 | 2 | ER+, PR+, HER2− | Y | N | 30 | Mammo | M & ALND | 0.7 | 3 | ER+, PR+, HER2− | ADF | 44 |
Abbreviations: ADF – alive disease free ALND – axillary lymph node dissection IDC – invasive ductal carcinoma L – lumpectomy M – mastectomy Mammo – mammographic presentation only ND – not determined
Figure III.
Distinct locations of a treatment scar and a recurrent tumor on MRI. (The circles denote the location of the initial and recurrent tumors.)
DISCUSSION
This prospective study of a carefully selected group of patients treated with 3D-APBI to a dose of 32 Gy in 8 twice-daily fractions with a median follow-up of nearly 6 years showed excellent overall local control, with a 5-year IBTR rate of 5% (1–10%). When evaluated by receptor phenotype, similarly to several WBI studies(7), it appears that patients with TN or ER-negative tumors are at higher risk of IBTR than are patients with other tumors. In this study, TN tumor status was the most significant variable predicting for the risk of an IBTR. Patients with such tumors had a 5-year actuarial risk of IBTR of 33%, compared to only 2% for other patients.
This finding is consistent with and extends that of a prior registry study of patients treated using balloon brachytherapy, in which ER negativity was the only variable significantly associated with IBTR, with an odds ratio of 4.01(9). Two other studies did not find TN phenotype to be associated with a higher rate of IBTR after treatment exclusively or predominantly with brachytherapy APBI, however these studies had substantially shorter lengths of follow-up and may have suffered from bias in their retrospective design(10, 11). One study of 209 women treated with brachytherapy to a dose of 34 Gy in 10 fractions from 2003 – 2009 at the Cancer Center of Irvine, with a median follow-up of 22 months, found only 3 patients experienced IBTR, with none amongst the 19 TN tumors(10). These results must be interpreted with caution, since the 5 local recurrences among patients with TN disease on our trial occurred between 30–55 months after therapy. The second study evaluated 202 women treated from 1999–2009 at the Beaumont Cancer Institute, of whom 20 had TN tumors and 182 had at least one receptor positive(11). Nearly half of all patients were treated with 3D-CRT (38.5 Gy in 10 fractions administered twice daily), with the remainder treated with interstitial brachytherapy (50 Gy with low dose-rate sources and 34 Gy in 10 fractions given twice daily with a high dose-rate technique) or balloon brachytherapy (34 Gy in 10 fractions). With a median follow-up of 4.1 years for the TN patients, none developed IBTR, regional nodal failure, or distant metastases. (The respective risks of these failures were 1.4%, 1.5%, and 2.8% for patients with at least one positive receptor, at a median follow-up of 5.1 years.) A grouped comparison of any recurrence-type showed no significant difference between the TN or receptor positive groups. The lack of any failures amongst the TN group, as well as the retrospective nature of the study, suggest a possible selection bias.
There are other possible explanations for the results of this study. Most of the TN patients with IBTR had chemotherapy prior to radiation, thus delaying the start of RT. A randomized study evaluating the importance of chemotherapy and radiation sequencing did not show that the sequencing of chemotherapy and RT affected local control for patients with margins wider than 1 mm(16). However, HER2 testing was not routinely performed at that time, and evaluation by TN status could not be done. Our findings suggest a possible benefit to early initiation of APBI for patients with TN tumors, which are likely to proliferate more rapidly than other phenotypes. It is also possible that TN and high-grade tumors have poorer outcomes following hypofractionated radiation compared to other schemes, as was found in the Canadian WBI hypofractionation trial for patients with grade 3 tumors(17). (There was also a nonsignificant trend for patients with ER-negative tumors to have increased IBTR with hypofractionated treatment.)
An important question relates to the location of the recurrences. Prior pathologic studies have shown the difficulty in correlating a clinical classification of a true recurrence relative to a molecular assessment of the clonality of initial and recurrent tumors(18). The recurrent tumors in our trial had the same receptor staining and similar morphology as the initial tumors, yet none of them recurred within 3 cm of the initial tumor site. A recent study evaluating the location of TN tumor recurrence following WBI showed it to occur more frequently in the area of the initial tumor than elsewhere in the breast(19). An explanation for this apparent discrepancy could relate to the relative tumor burden remaining in a breast following a lumpectomy. The tumor burden outside the APBI volume is likely to be small, making it possible that the radiation dose given with WBI is sufficient to eradicate it. It may be that the residual tumor burden within the boost field is sufficiently large that it cannot be eradicated by WBI, despite the higher dose administered there. Viewed in another way, APBI may be eradicating disease in the treatment area, or delaying the time at which it would re-appear, thus allowing more distant disease to manifest first. It is also possible that patients with recurrent tumors had multifocal or multicentric disease at presentation, and that breast MRI could have aided avoidance of these IBTRs(20). Taken together, we believe the results of this trial suggest that 32 Gy of 3D-APBI offered tumor control within the irradiated volume at nearly 6 years of median follow-up, but that despite the careful exclusion criteria of the trial, certain tumors will recur within the breast, and TN tumors appear to be at greater risk of this. With continued follow-up of this patient cohort, future analyses may answer whether tumors of different receptor phenotypes have differing temporal recurrence patterns.
Our data must be interpreted in the context of the study design, with the limited sample size and number of events weighed against the relatively recent yet increasingly broad application of 3D-APBI. Ongoing and future 3D-APBI trials of larger sample size and longer follow-up should continue to assess the suitability of patients for treatment, with receptor phenotype likely to remain an important variable. While recognizing this study's limitations, we believe its outcomes should both enhance confidence in the efficacy of 3D-APBI for patients with ER-positive tumors and serve a precautionary note in its use for treatment of patients with TN breast cancer. Current guidelines include ER-negative patients in a “cautionary” group(6), but allow the treatment of patients with all receptor phenotypes with 3D-APBI. Our data suggest that those with TN breast cancer may be at significantly elevated risk of IBTR following treatment. Larger, prospective 3D-APBI clinical trials with long follow-up should continue evaluating the effect of hormone receptor phenotype on IBTR rates. Randomized trials comparing APBI and WBI (such as NSABP-B39 / RTOG-0413) will reveal whether outcomes differ by volume of irradiation.
Summary.
98 patients with Stage I breast cancer were prospectively treated with 3D-conformal accelerated partial breast irradiation (3D-APBI) using 32 Gy with 8 twice-daily fractions. The overall 5-year actuarial IBTR rate was 5% (95% CI, 1–10%). The rate was 33% (0–57%) in patients with triple-negative (TN) disease and 2% (0–6%) in non-TN patients (p<0.0001). Larger, prospective 3D-APBI clinical trials should continue evaluating the effect of receptor phenotype on IBTR rates.
Acknowledgments
This work was supported in part by awards from the National Cancer Institute (NCI) at the National Institutes of Health (NIH) (R01CA139118 and P50CA089393 to A.G.T.). Content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 52nd Annual Meeting of the American Society of Therapeutic Radiology and Oncology, October 31, 2010–November 4, 2010, San Diego, CA.
Conflicts of Interest Notification The authors report no actual or potential conflicts of interest.
REFERENCES
- 1.Beitsch PD, Shaitelman SF, Vicini FA. Accelerated partial breast irradiation. J Surg Oncol. 2011;103:362–368. doi: 10.1002/jso.21785. [DOI] [PubMed] [Google Scholar]
- 2.Berrang TS, Olivotto I, Kim DH, et al. Three-year outcomes of a canadian multicenter study of accelerated partial breast irradiation using conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:1220–1227. doi: 10.1016/j.ijrobp.2010.07.2003. [DOI] [PubMed] [Google Scholar]
- 3.Vicini F, Winter K, Wong J, et al. Initial efficacy results of RTOG 0319: three-dimensional conformal radiation therapy (3D-CRT) confined to the region of the lumpectomy cavity for stage I/ II breast carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:1120–1127. doi: 10.1016/j.ijrobp.2009.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polgar C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma--5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694–702. doi: 10.1016/j.ijrobp.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
- 6.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 8.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 9.Shaitelman SF, Vicini FA, Beitsch P, et al. Five-year outcome of patients classified using the American Society for Radiation Oncology consensus statement guidelines for the application of accelerated partial breast irradiation: an analysis of patients treated on the American Society of Breast Surgeons MammoSite Registry Trial. Cancer. 2010;116:4677–4685. doi: 10.1002/cncr.25383. [DOI] [PubMed] [Google Scholar]
- 10.Wilder RB, Curcio LD, Khanijou RK, et al. Results with accelerated partial breast irradiation in terms of estrogen receptor, progesterone receptor, and human growth factor receptor 2 status. Int J Radiat Oncol Biol Phys. 2010;78:799–803. doi: 10.1016/j.ijrobp.2009.08.081. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson JB, Reid RE, Shaitelman SF, et al. Outcomes of breast cancer patients with triple negative receptor status treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;81:e159–164. doi: 10.1016/j.ijrobp.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Recht A, Ancukiewicz M, Alm El-Din MA, et al. Lung dose-volume parameters and the risk of pneumonitis for patients treated with accelerated partial-breast irradiation using three-dimensional conformal radiotherapy. J Clin Oncol. 2009;27:3887–3893. doi: 10.1200/JCO.2008.20.0121. [DOI] [PubMed] [Google Scholar]
- 13.King TA, Bolton JS, Kuske RR, et al. Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for T(is,1,2) breast cancer. Am J Surg. 2000;180:299–304. doi: 10.1016/s0002-9610(00)00454-2. [DOI] [PubMed] [Google Scholar]
- 14.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 15.Kozak KR, Smith BL, Adams J, et al. Accelerated partial-breast irradiation using proton beams: initial clinical experience. Int J Radiat Oncol Biol Phys. 2006;66:691–698. doi: 10.1016/j.ijrobp.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 16.Bellon JR, Come SE, Gelman RS, et al. Sequencing of chemotherapy and radiation therapy in early-stage breast cancer: updated results of a prospective randomized trial. J Clin Oncol. 2005;23:1934–1940. doi: 10.1200/JCO.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 17.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 18.McGrath S, Antonucci J, Goldstein N, et al. Long-term patterns of in-breast failure in patients with early stage breast cancer treated with breast-conserving therapy: a molecular based clonality evaluation. Am J Clin Oncol. 2010;33:17–22. doi: 10.1097/COC.0b013e31819cccc3. [DOI] [PubMed] [Google Scholar]
- 19.Hattangadi-Gluth JA, Wo JY, Nguyen PL, et al. Basal subtype of invasive breast cancer is associated with a higher risk of true recurrence after conventional breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2012;82:1185–1191. doi: 10.1016/j.ijrobp.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Hallaq HA, Mell LK, Bradley JA, et al. Magnetic resonance imaging identifies multifocal and multicentric disease in breast cancer patients who are eligible for partial breast irradiation. Cancer. 2008;113:2408–2414. doi: 10.1002/cncr.23872. [DOI] [PubMed] [Google Scholar]