Abstract
The entorhinal cortex is a brain area with multiple reciprocal connections to the hippocampus, amygdala, perirhinal cortex, olfactory bulb and piriform cortex. As such, it is thought to play a large role in the olfactory memory process. The present study is the first to compare lateral entorhinal and anterior piriform cortex odor-evoked single-unit and local field potential activity in mouse. Recordings were made in urethane-anesthetized mice that were administered a range of 3 pure odors and 3 overlapping odor mixtures. Results show that spontaneous as well as odor-evoked unit activity was lower in lateral entorhinal versus piriform cortex. In addition, units in lateral entorhinal cortex were responsive to a more restricted set of odors compared to piriform. Conversely, odor-evoked power change in local field potential activity was greater in the lateral entorhinal cortex in the theta band than in piriform. The highly odor-specific and restricted firing in lateral entorhinal cortex suggests that it may play a role in modulating odor-specific, experience- and state-dependent olfactory coding.
Keywords: Olfaction, Piriform cortex, Lateral entorhinal cortex, Single-Unit, Mouse
INTRODUCTION
The entorhinal cortex (EC) is the major gateway for information entering the hippocampal formation. The EC is a component of the medial temporal lobe memory system (Bunsey and Eichenbaum, 1993, Young et al., 1997, Wirth et al., 1998b, Mayeaux and Johnston, 2004), though it is increasingly believed to have perceptual functions (Baxter, 2009, Suzuki, 2009). Neuroanatomically, the EC is transitional between paleocortex and neocortex. For example, similar to piriform cortex (PCX), but different from neocortex, the EC receives extensive afferent input to Layer I. Similar to neocortex but different from PCX, the EC is organized into six layers, each with differing local and network connections. In addition to connections with the hippocampus, the EC also receives dense input from the perirhinal cortex, amygdala, thalamus, and modulatory areas like the cholinergic medial septum (Sewards and Sewards, 2003, Kerr et al., 2007, Canto et al., 2008). Furthermore, EC displays intrinsic memory functions, for example maintaining stimulus specific neural activity during delay periods (Young et al., 1997, Hasselmo and Brandon, 2008, Yoshida et al., 2008). Finally, the EC appears uniquely sensitive to a number of disorders including Alzheimer’s disease (Braak and Braak, 1992), with Layer II neurons particularly vulnerable (Stranahan and Mattson, 2010).
In terms of olfaction, the EC (primarily the lateral EC [LEC]) receives direct input from the main olfactory bulb and is thus considered part of the olfactory cortex (Haberly and Price, 1978, Cleland and Linster, 2003, Agster and Burwell, 2009, Sosulski et al., 2011). It also receives input from the piriform cortex (PCX), and projects directly back to both areas (Haberly and Price, 1978, Cleland and Linster, 2003, Agster and Burwell, 2009, Sosulski et al., 2011). In fact in rodents, afferent fibers from olfactory areas are the dominant input to LEC (Kerr et al., 2007). This input terminates in Layer I on the apical dendrites of Layer II/III pyramidal and stellate cells (Luskin and Price, 1983, Burwell and Amaral, 1998b). These Layer II/III neurons are also the main class of output neurons to both the hippocampal formation and back to olfactory areas (Agster and Burwell, 2009). Thus, given the LEC’s connectivity, it may serve as an important top-down control over piriform cortex function.
However, despite fitting the classic definition of an olfactory cortical structure (direct target of olfactory bulb mitral cells) very little is known about LEC sensory physiology. LEC neurons are responsive to objects in an open field (Deshmukh and Knierim, 2011), and to odors (Boeijinga and Lopes da Silva, 1989, Eeckman and Freeman, 1990, Young et al., 1997, Kay and Freeman, 1998, Chabaud et al., 2000, Petrulis et al., 2005), but no direct comparison of LEC olfactory processing with activity in PCX has been performed. The present study is a first step to gain insight into the function of the LEC in olfactory processing by exploring odor-evoked single-unit and local field potential activity in this area in urethane-anesthetized mice and comparing it with activity in the anterior PCX (aPCX).
EXPERIMENTAL PROCEDURE
Subjects
A total of 27 B6sJLF/J (22–38g) mice obtained from Jackson Laboratories and a breeding colony at the Nathan Kline Institute were used in the present study. Lateral entorhinal cortex units (LEC) were obtained from 14 mice while anterior piriform cortex (aPCX) units were obtained from the remaining 9 mice. Only 1 region was recorded from in each of these animals. In 4 animals, simultaneous LFP recordings were performed in both aPCX and LEC. All animals were group-housed ranging in groups of 3 to 4 animals in polypropylene cages. Food and water was available ad lib. All handling, housing, and experimental procedures were in accordance with the Institutional Animal Care and Use Committee guidelines at Nathan Kline Institute and NIH guidelines for the proper treatment of animals.
Experimental design
The present experiment sought to determine the response of single units and local field potentials in the aPCX and LEC to a series of 3 monomolecular odorants and 3 odorant mixtures. Animals were anesthetized and each odor was randomly administered 4 times (24 total administrations) for 2 s with at minimum a 30 s interstimulus interval and the same odor was never administered consecutively.
Recording and odorant stimulation
Single-unit recording procedures for the aPCX and LEC were performed similar to previous reports (Wilson, 1998, Kadohisa and Wilson, 2006a). Briefly, animals were anesthetized with urethane (1.25 g/kg). Respiration was monitored throughout the recording session with a piezoelectric device placed under the animal’s chest. Single units were recorded using a tungsten microelectrode (1–5 Mohm and signals were acquired and analyzed with Spike2 (CED). Individual units were identified by having at least a 2-ms refractory period in interval histograms. Layer II/III aPCX units (filtered 0.3–3 kHz) were identified by OB evoked responses and histological confirmation while LEC units were identified by histological confirmation. LFPs (filtered at 0.1–300 Hz) were recorded with the same electrode at the same time as the single units.
Stimuli were delivered with a flow-dilution olfactometer that was positioned 2 cm from the animal’s nose. Odor vapor was added with a computer-controlled solenoid at a rate of 0.1 liters per minute (LPM) to a constant flow of nitrogen gas (N2) at 1 LMP. Odors were administered for 2 s per trial with at least a 30 s interstimulus interval. Given that most natural odors are complex mixtures, and that monomolecular odorants and mixtures may be processed differently in higher order cortical areas, we chose to use both monomolecular odorants and well characterized odorant mixtures. The stimuli used were as follows: ethyl valerate, isoamyl acetate, heptanal, and 3 mixtures termed 10c, 10c-1, and 10cR1. Components of the mixture and how each differed have been previously detailed (Barnes et al., 2008, Chapuis and Wilson, 2012, Lovitz et al., 2012). Briefly, 10c is comprised 10 components: isoamyl acetate, nonane, ethyl valerate, 5-methyl-2-hexanone, isopropylbenzene, 1-pentanol, 1,7-octadiene, 2-heptanone, heptanal, and 4-methyl-3-penten-2-one. Isoamyl acetate is removed from the mixture to create 10c-1 and it is replaced by limonene in 10cR1. Both pure odorants and components of odorant mixtures were diluted in mineral oil to a concentration of 100 ppm based on vapor pressure. Thus, mixtures were at a higher total concentration than pure odorants.
Data Analysis
Single unit identification and analyses were all performed using Spike2 using template matching and principal component analyses. The recordings were verified as coming from single-units by confirming at least a 2 ms refractory period in interval histograms. Responses to the different odor stimuli were analyzed by comparing spike activity 3 seconds before stimulus onset and 3 s after stimulus onset. Odor-evoked response magnitudes within individual neurons were normalized to the response of the odor evoking the maximal response in that cell (best odor) to obtain a relative response magnitude to each odor for each neuron. Spontaneous activity was calculated to be the per-second average of the pre-stimulus activity across 6 odors. Due to very low spontaneous activity rates in aPCX and LEC, individual single-unit responses were not classified in to categories such as suppressive. However, mean receptive fields displayed relative decreases in activity from baseline consistent with suppression.
Odor-evoked LFP oscillatory responses were estimated in the theta (7–12 Hz), beta (15–35 Hz) and gamma (40–80 Hz) frequency bands. Power spectra for these responses were calculated by comparing activity 3 s before stimulus onset and 3 seconds from initial stimulus onset. Odor-evoked oscillatory power was determined by dividing oscillatory power during the 3 sec odor period by baseline pre-odor activity.
All statistical analyses were done using StatView. Repeated measures and one-way ANOVAs were used to compare odor-evoked and spontaneous activity between aPCX and LEC single units respectively. In addition post hoc Fisher tests and t-tests were used to compare odor-evoked responses of different odors. For LFP data, two-way ANOVAs were used to compare spontaneous activity as well as odor-evoked activity across frequency bands in LEC and aPCX. Post-hoc Fisher tests were then (Kay and Freeman, 1998) used for appropriate pair-wise comparisons. For respiration entrainment data, MatLab software was used to determine significance of individual unit activity phase relationship with the respiratory cycle using Rayleigh statistics over a duration of at least 10 minutes. Similarly, for phase locking data, MatLab software was used to determine the degree, vector strength, and significance of beta phase locking to respiration.
Histology
After recording, mice were overdosed with urethane and transcardially perfused with saline and 4% (wt/vol) formaldehyde. Coronal brain sections (40 µm thick) were cut using a microtome (Leica), mounted, and stained with cresyl violet to identify electrode positioning in aPCX and LEC.
RESULTS
Single Unit Spontaneous and Odor Evoked Activity in aPCX and LEC
A total of 42 LEC single units obtained from 13 mice and 39 aPCX units obtained from 9 mice were used in the present analyses. Electrode tip positions in LEC and aPCX both are show in Fig. 1a and 1b respectively. In addition, a representative recording of an LEC single-unit odor-evoked response is shown in Fig. 2.
Figure 1.
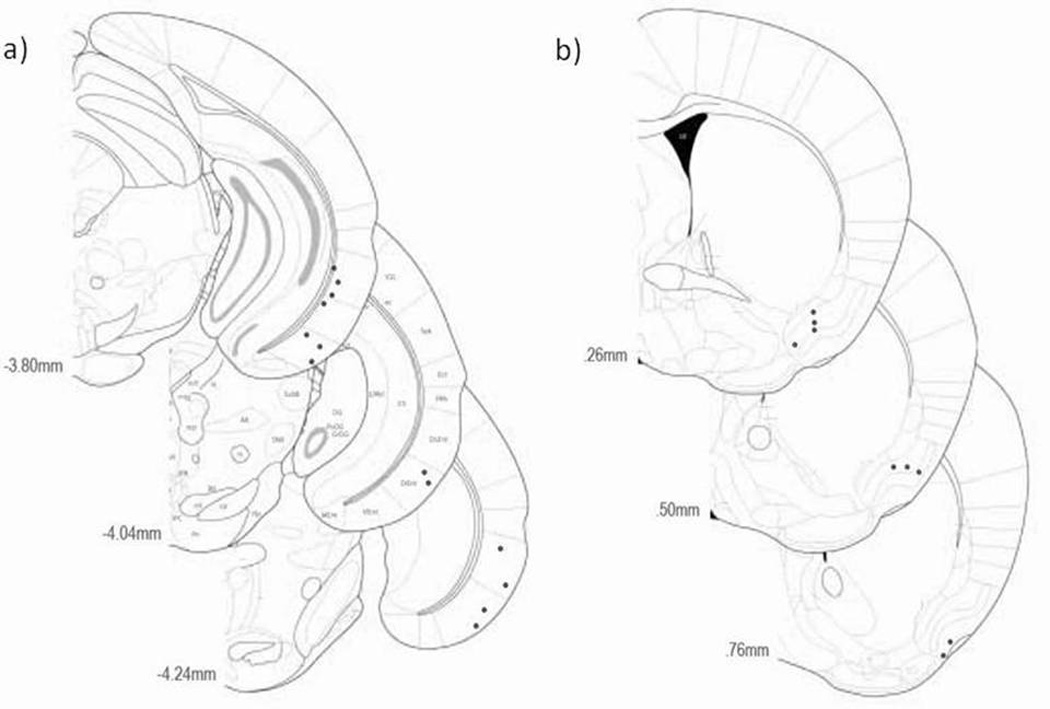
Electrode placement in the a) LEC of 13 animals and b) aPCX of 9 animals. Coronal stereotaxic images showing the approximate placement of the final tip location of recording electrode tracks (black dots). Images adapted from (Franklin and Paxinos, 2008).
Figure 2.
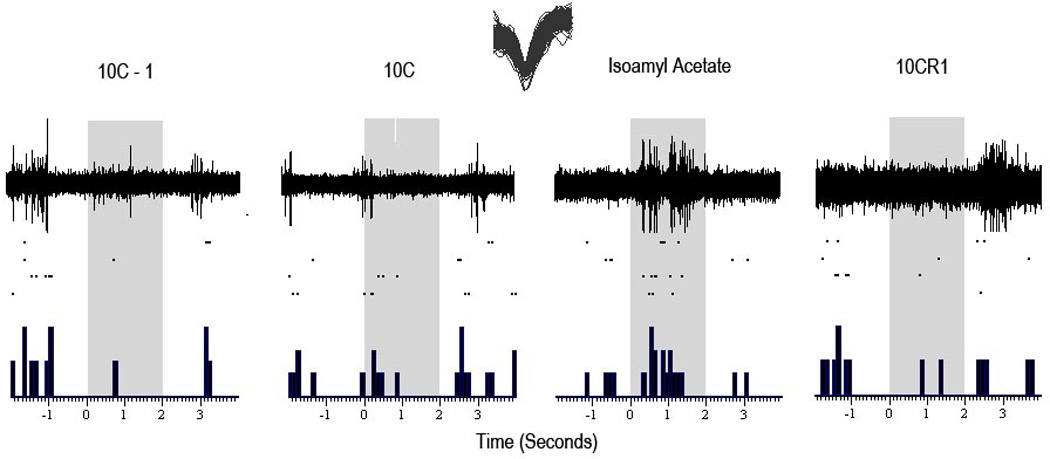
Representative odorant responsivity to 4 sample odors in a single unit in the LEC from a single mouse. The top half shows waveform activity in response to a single presentation of the named odorant. The bottom half shows raster plots (dots) of responsivity to each presentation of an odor. Rasterplots and peristimulus time histograms (PSTH) show reliability of responses. Data for rasters and PSTHs obtained by extraction of single-unit activity from the raw recordings (see Methods). Overlapping single-unit waveforms shown on top. Odorant presentation time is enclosed in the shaded area starting from 0 seconds to 2 seconds.
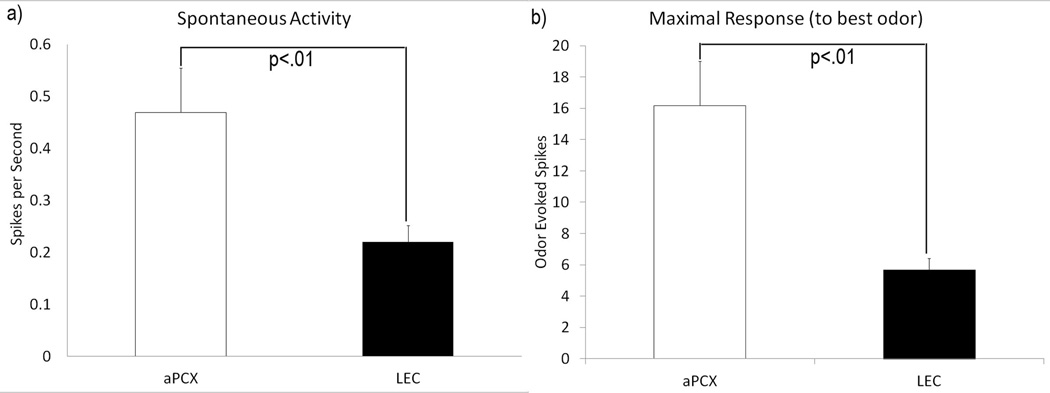
In urethane anesthetized mice, single-unit spontaneous activity in LEC was significantly lower than in aPCX (F(1, 79) = 7.83, p <.01) (Figure 3a). Similarly, maximal single-unit odor-evoked activity (mean maximal odor-evoked response = spike count during 3 sec post odor onset – spike count during 3 sec pre-odor onset) was significantly lower in LEC single-units than aPCX units (F(1, 79) = 13.73, p <.001) (Fig. 3b). In addition, a simple comparison was made to determine whether aPCX or LEC neurons were maximally excited by monomolecular odorants or by mixtures. For cells that showed a maximal evoked response to a single stimulus (aPCX, n = 39; LEC, n = 38), 42% (16/38) of units in LEC had a maximal response to monomolecular (pure) odorants while 58% (22/38) had a maximal response to odorant mixtures. Meanwhile 28% (11/39) of units in aPCX had a maximal response to monomolecular odorants while 62% (28/39) had a maximal response to odorant mixtures. Chi-square analyses showed a non-significant effect of type of odorant on maximal odor-evoked single unit response occurrence between the aPCX and LEC (χ2(3) = 1.63, p = N.S.).
Figure 3.
Spontaneous activity (a) and the maximal odor-evoked response to the best odor (b) in aPCX versus LEC. Both spontaneous activity (p <.01) and aPCX maximal odor-evoked responses (p < .001) were significantly greater than those in LEC. Error bars represent ± 1 SEM.
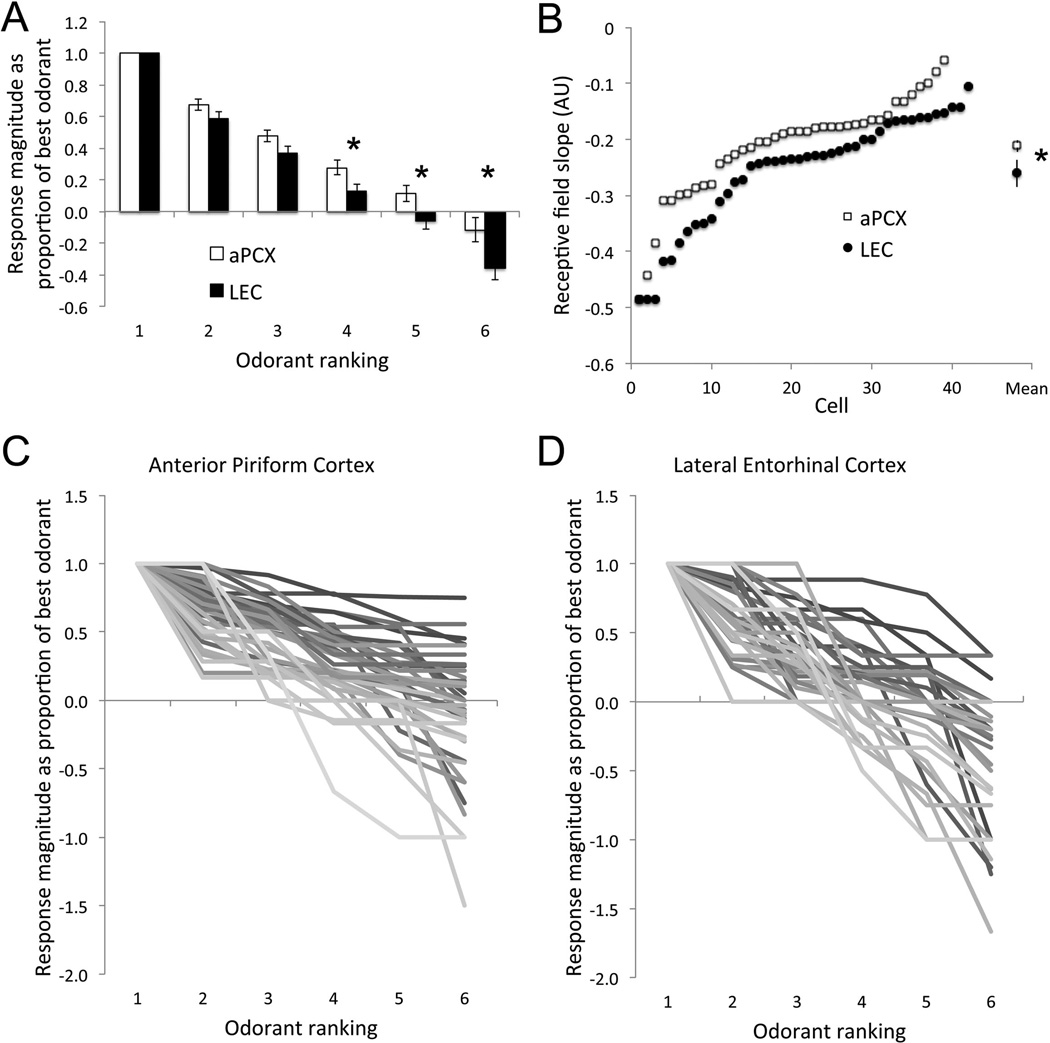
In order to investigate the specificity with which units in LEC and aPCX responded to the different stimuli, odor-receptive fields normalized to the best odor were compared with a two-way (region × odorant) repeated measures ANOVA with normalized response to different odors as the repeated measure. aPCX units were significantly more broadly tuned than LEC units across odors (F(1,79) = 7.79, p <.01), post-hoc (p< .05). On average, aPCX neurons showed net excitatory responses (at least 30% change in firing rate from baseline) to 5 of 6 odors tested while LEC single-units were excited by only 3, and showed significantly more robust net suppression than aPCX (Fig. 4a).
Figure 4.
a) Single-unit odor receptive fields in the aPCX are significantly more broad than in LEC. Responses are normalized to best odor within each cell. Therefore, a measure of 1 is the response to the best odor with subsequent responses being expressed as a percentage of that response strength. The strength of LEC responses to the fourth, fifth, and worst odors was significantly lower than matched responses in the aPCX (p<.05). Error bars represent ± 1 SEM. b) Receptive field slopes in aPCX and LEC plotted for each unit recorded. Here, aPCX cells demonstrate a reduced slope compared LEC units. Response magnitude of each unit to each odor in c) aPCX and d) LEC. aPCX units are much more broadly tuned compared to LEC.
To further examine receptive field breadth, the slope of the receptive field from best odor to worst odor was calculated for each single-unit (Fig. 4b) and compared across regions. As can be seen in the plot of all receptive fields (Fig 4c and d) and their slopes (Fig. 4b), LEC receptive field slopes were significantly greater than aPCX unit receptive field slopes (t-test, p < 0.05), consistent with more narrow tuning in LEC.
Single-unit Respiration Entrainment
In order to see if unit activity in LEC and aPCX were associated with respiration patterns, phase plots of unit activity across the respiratory cycle were built for each cell and the significance of phase locking was determined with Rayleigh statistics. A Chi-square test was used to compare the proportion of units firing in phase with respiration in each area. 31 units were assessed in LEC (10 units were lost due to lack of respiration data) and 39 units were assessed in aPCX. In total, 19% of units were respiration linked in LEC (6 of 31) while 74% of units were respiration linked in aPCX (29 of 39), (χ2(1) = 20.9, p <.001) (Fig 5a,b).
Figure 5.
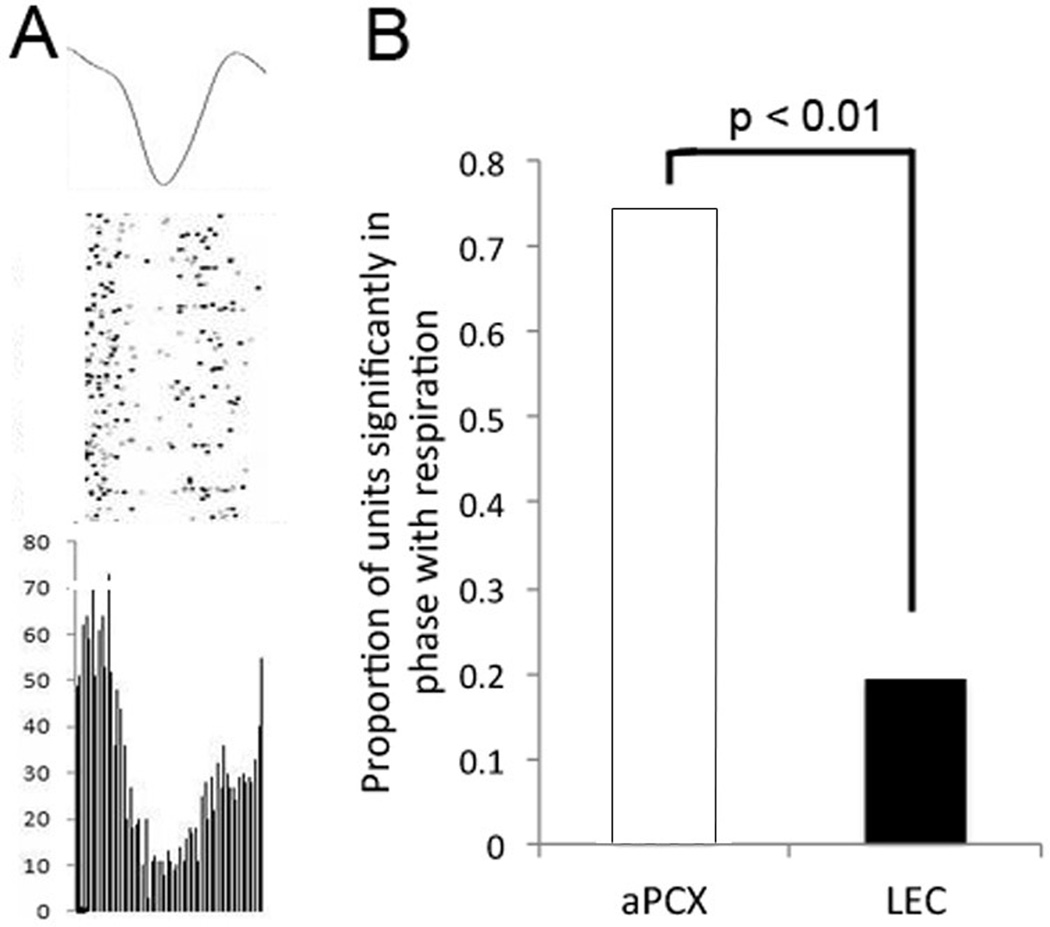
Respiration entrainment in LEC vs. aPCX units. a) Averaged respiration waveform, aPCX single-unit activity displayed as rasters (middle) and as a phase plot (bottom) relative to the respiratory cycle. This unit showed highly significant entrainment to the respiratory cycle as assessed with Rayleigh statistics. b) The proportion of units firing in phase with respiration in the LEC and aPCX).Significantly more aPCX units fired in phase with respiration compared to LEC.
Odor Evoked Local Field Potentials
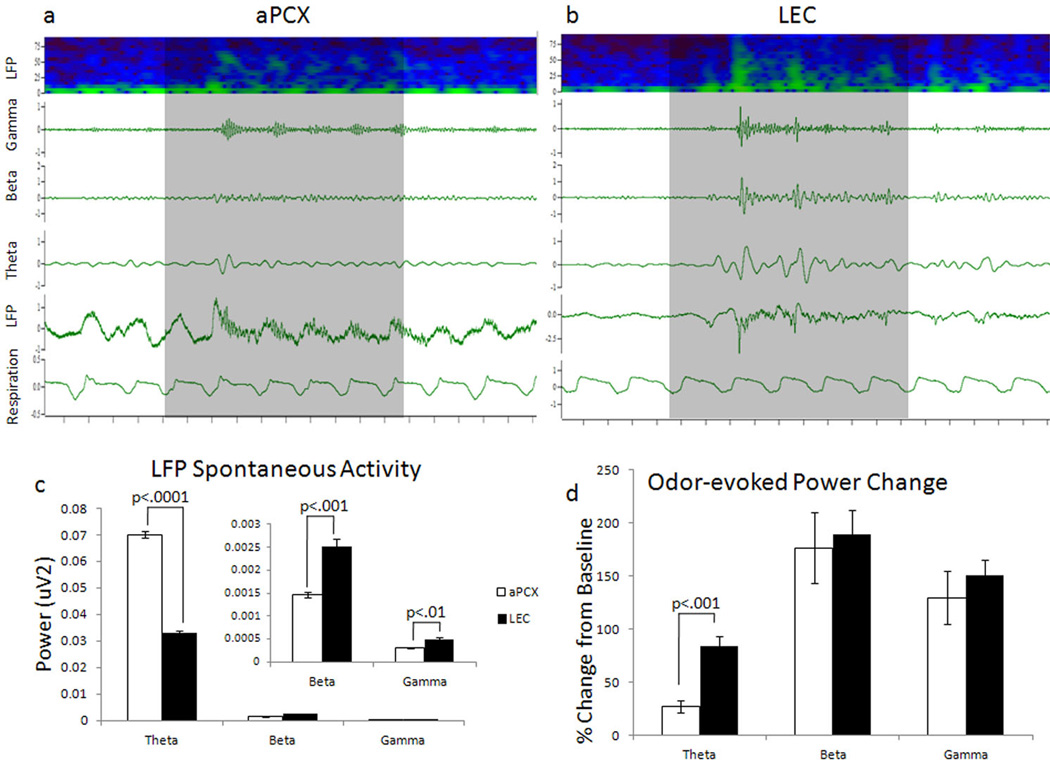
No difference was observed between LFP data obtained with simultaneous aPCX and LEC recordings and those obtained when recording a single location, thus the data are combined for these analyses. A comparison of spontaneous LFP oscillatory activity in aPCX (Fig. 6a) and LEC (Fig. 6b) in the theta, beta, and gamma band frequency band revealed that there were significant main effects of both brain region (F(1, 30) = 675.72, p < .0001) and frequency band (F(2, 30) = 5326.71, p < .0001), as well as an interaction of the two (F(2, 30) = 747.19, p < .0001). Post-hoc comparisons demonstrated that there was significantly greater spontaneous theta power (p<.0001) in aPCX compared to LEC, while beta (p<.001), and gamma (p<.01) band power was greater in LEC than aPCX (Fig. 6c).
Figure 6.
Representative field potential recordings from a) aPCX and b) LEC showing LFP as a sonogram and waveform as well as individually digitally filtered theta, beta, and gamma components. Odorant stimulation duration (2 sec.) is depicted by the shaded area. Odors evoked enhanced oscillatory activity in LEC compared to the aPCX. c) Spontaneous LFP activity in aPCX and LEC. While theta band power was significantly higher in aPCX compared to LEC, beta and gamma band spontaneous activity were higher in LEC than in aPCX. d) Odor-evoked oscillations in the aPCX and LEC across all odors. LEC evoked oscillations were significantly greater than aPCX specifically within the theta band. Error bars represent ± 1 SEM.
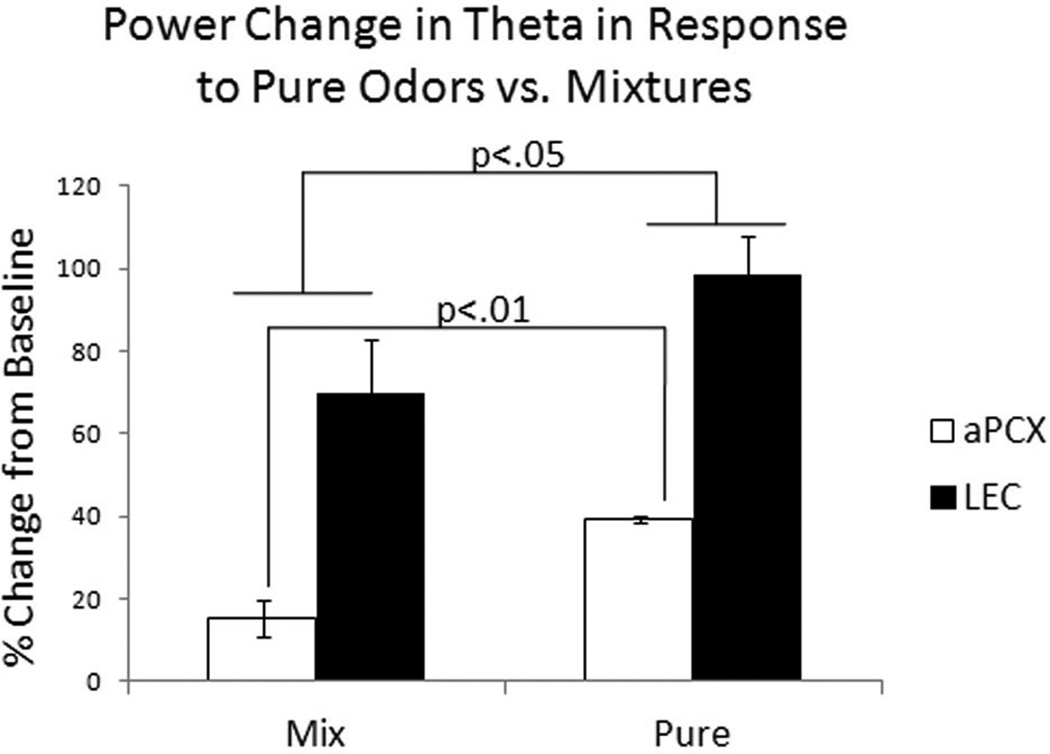
Relative odor evoked oscillatory LFP activity was enhanced in the LEC compared to the aPCX, though this effect was limited to the theta band. For analysis of odor-evoked activity, the odor-evoked LFP power change was calculated as the ratio of the power 3 seconds following odorant onset over the spontaneous activity 3 seconds prior to odorant onset. This measure was then averaged across all odorants to determine whether there was a difference in odor-evoked LFP between LEC and aPCX. There was a significant main effect of frequency band (F(1, 30) = 19.53, p <.0001), and nearly significant main effect of brain region (F(1,30) = 3.22, p = 0.08), with no interaction between frequency and brain region (F(2,30) = 0.63, p = 0.53) (Fig. 6d). Post-hoc comparisons revealed significantly greater odorant-evoked theta band oscillations in LEC than in aPCX. The LEC LFP response was larger than the aPCX in response to both pure and odor mixtures (F(1, 8) = 44.79, p < .0005), and pure odorants elicited a greater response compared to odor mixtures in both regions (F(1,8) = 9.57, p <.05) (Fig. 7). Finally, while there was no significant difference in power change in response to pure or odor mixtures in the LEC. Pure odors elicited a greater response in theta than mixtures in aPCX (F(1,4) = 29.03, p<.005). These results are consistent with mixture suppression effects reported throughout the olfactory system (Kadohisa and Wilson, 2006b).
Figure 7.
Power change in odor-evoked theta oscillations recorded in LEC and aPCX in response to pure, monomolecular odors and odor mixtures. Pure odors elicited a significantly larger power change than odor mixtures in aPCX (p<.01) though this difference was absent in LEC. In addition, pure odors elicited a larger power change than mixtures in both LEC and aPCX (F(1, 116) = 4.09, p <.05). Furthermore, LEC theta power change in response to both types of odors was greater than in aPCX (F(1, 116) = 12.14, p <.001).
LFP Phase Locking with Respiration
Due to previous observations that odor-evoked frequency activity occurs during specific phases of the respiration cycle (Buonviso et al., 2003), beta frequency phase locking to respiration was explored in aPCX and LEC (Fig. 8a). In animals with dual aPCX and LEC recordings, individual beta band oscillations were extracted through thresholding of the beta frequency filtered (15–35 Hz) LFPs, with the threshold amplitude set at twice the standard deviation of basal activity. Beta events were then compared to respiration, with respiration events set at the transition between inhalation and exhalation. There was significant entrainment of aPCX beta oscillations with respiration. However, there was no significant beta oscillation entrainment to respiration detected in the mean data from LEC (Fig. 8b). In analysis of individual animals, beta frequency was significantly related to the respiration cycle in 4 out of 4 animals in the aPCX but only 2 out of 4 animals in LEC (individual Rayleigh tests) (Fig bc).
Figure 8.
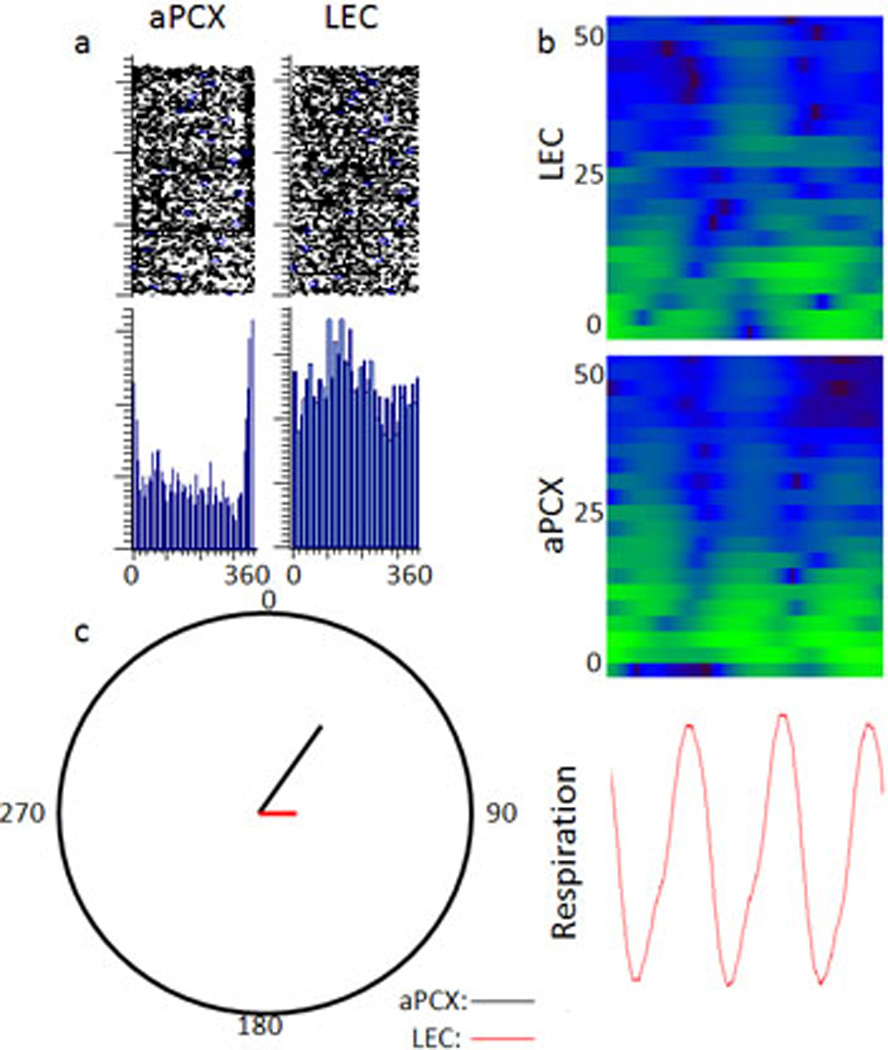
a) Raster plots of spontaneous and odor evoked beta waves in aPCX and LEC of a representative dual recording animal are shown in the top panels with respiration phase shown in the bottom panel. Note the high correlation of beta activity to respiration in aPCX and the weaker correlation in LEC. The transition between inhalation and exhalation occurs at time 0. b) Sonogram of oscillatory activity in LEC and aPCX relative to respiration (bottom panel) through a 1 second time span. Beta activity occurred consistently in phase with respiration in aPCX while not being phase locked to any phase of respiration in LEC. c) Mean computed vector length and angle across all 4 animals (transition between inhalation and exhalation occurs at angle 0). Note circle radius is 0.5.
DISCUSSION
The present study was an initial investigation into the role of the LEC in olfactory processing by recording odor-evoked single-unit and LFP activity. The data show that as compared to aPCX, LEC single-units had a lower spontaneous activity rate as well as lower maximal odor-evoked activity. In addition, single-units in LEC were more narrowly tuned to monomolecular odorants and mixtures than aPCX units, with fewer odorants evoking excitatory activity, and more commonly evoked a net suppression. The narrow tuning in the anesthetized mouse LEC is similar to that reported in the awake rat LEC (Young et al., 1997, Petrulis et al., 2005), and similar to differences reported between the anterior and posterior PCX (Litaudon et al., 2003). In addition to reduced odor responsiveness, LEC cells were also less likely to fire in phase with respiration than aPCX units, again similar to that reported in posterior PCX (Litaudon et al., 2003). In contrast, odor-evoked LFP oscillations in LEC were more robust than those in aPCX, though only in the theta frequency band.
The fact that LEC single-units showed weaker odor-evoked responses than aPCX and, yet LEC odor-evoked LFP theta activity was stronger than aPCX activity is interesting. This apparent disconnect between single-unit and LFP activity could derive from a number of mechanisms, including potential relative differences in inhibitory currents or other subthreshold events contributing to LFPs in the two areas. LEC LFP’s presumably also reflect activity in a several cortical layers, while aPCX LFP’s derive from a much simpler underlying neural architecture. Laminar current source density analyses of odor-evoked LEC activity, similar to that performed in piriform cortex (Ketchum and Haberly, 1993, Neville and Haberly, 2003) may help clarify this issue.
Odor specific activity in the LEC may have two functions. First, odor-specific responses in LEC neurons may be especially important given the apparent role of the EC in odor working memory (Young et al., 1997, Egorov et al., 2002, Hasselmo and Brandon, 2008), and as a major input to hippocampal circuits involved in other forms of odor memory (Staubli et al., 1984, Eichenbaum, 2004). Second, however, the LEC is not only a major afferent to the hippocampus, but is also a major descending input back to more peripheral regions of the olfactory pathway, such as the PCX and olfactory bulb (Insausti et al., 1997, Burwell and Amaral, 1998a, Cleland and Linster, 2003, Kerr et al., 2007, Wilson and Sullivan, 2011). The LEC appears to have a general suppressive effect on the olfactory bulb and PCX, as aspiration lesions of the EC can enhance odor-evoked c-fos activity in both the OB and PCX (Bernabeu et al., 2006), and electric stimulation of EC can suppress PCX evoked potentials driven by OB stimulation (Mouly and Di Scala, 2006). In fact, EC lesions can enhance acquisition of simple olfactory Go-No-Go tasks (with molecularly distinct odors) and simple odor aversion learning (Otto et al., 1991, Ferry et al., 1996, Wirth et al., 1998a). However, these previous studies have removed or activated much if not all of the LEC simultaneously. The present results, showing that individual odors evoke activity in only a small population of narrowly tuned cells (relative to the aPCX), suggests that LEC feedback could be highly odor specific. Given the access of LEC neurons to inputs from hippocampus, amygdala, perirhinalcortex and basal forebrain, this suggest that the LEC could serve as an odor specific, experience- and state-dependent modulator of olfactory coding. In fact, top-down EC driven-beta oscillations recorded in the olfactory bulb have been shown to occur pre-odor stimulus onset in difficult tasks (Kay and Freeman, 1998). The LEC, thus may be ideally situated to shape odor processing and perception (Chen et al., 2011, Chapuis and Wilson, 2012). Further exploration of the sensory physiology of identified LEC neurons and in a variety of tasks is ongoing.
Highlights.
-
-
LEC units had less spontaneous and lower odor-evoked activity than aPCX units
-
-
LEC units responded to a more restricted odor set than aPCX units.
-
-
While aPCX units were significantly phase-locked to respiration, LEC units were not.
-
-
LEC odor-evoked LFP power change was greater than aPCX in the theta frequency.
-
-
There was significant entrainment of aPCX but not LEC beta oscillations with respiration.
ACKNOWLEDGEMENTS
This work was supported by grants DC003906 and AG037693 to D.A.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agster KL, Burwell RD. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus. 2009;19:1159–1186. doi: 10.1002/hipo.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DC, Hofacer RD, Zaman AR, Rennaker RL, Wilson DA. Olfactory perceptual stability and discrimination. Nature neuroscience. 2008;11:1378–1380. doi: 10.1038/nn.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG. Involvement of medial temporal lobe structures in memory and perception. Neuron. 2009;61:667–677. doi: 10.1016/j.neuron.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Thiriet N, Zwiller J, Di Scala G. Lesion of the lateral entorhinal cortex amplifies odor-induced expression of c-fos, junB, and zif 268 mRNA in rat brain. Synapse. 2006;59:135–143. doi: 10.1002/syn.20224. [DOI] [PubMed] [Google Scholar]
- Boeijinga PH, Lopes da Silva FH. Modulations of EEG activity in the entorhinal cortex and forebrain olfactory areas during odour sampling. Brain Res. 1989;478:257–268. doi: 10.1016/0006-8993(89)91506-0. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neurosci Res. 1992;15:6–31. doi: 10.1016/0168-0102(92)90014-4. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Critical role of the parahippocampal region for paired-associate learning in rats. Behav Neurosci. 1993;107:740–747. doi: 10.1037//0735-7044.107.5.740. [DOI] [PubMed] [Google Scholar]
- Buonviso N, Amat C, Litaudon P, Roux S, Royet JP, Farget V, Sicard G. Rhythm sequence through the olfactory bulb layers during the time window of a respiratory cycle. Eur J Neurosci. 2003;17:1811–1819. doi: 10.1046/j.1460-9568.2003.02619.x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998a;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. The Journal of Comparative Neurology. 1998b;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Canto CB, Wouterlood FG, Witter MP. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast. 2008;2008 doi: 10.1155/2008/381243. 381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud P, Ravel N, Wilson DA, Mouly AM, Vigouroux M, Farget V, Gervais R. Exposure to behaviourally relevant odour reveals differential characteristics in rat central olfactory pathways as studied through oscillatory activities. Chem Senses. 2000;25:561–573. doi: 10.1093/chemse/25.5.561. [DOI] [PubMed] [Google Scholar]
- Chapuis J, Wilson DA. Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nature neuroscience. 2012;15:155–161. doi: 10.1038/nn.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Barnes DC, Wilson DA. Generalized versus stimulus-specific learned fear differentially modifies stimulus encoding in primary sensory cortex of awake rats. Journal of Neurophysiology. 2011;106:3136–3144. doi: 10.1152/jn.00721.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Linster C. Central olfactory structures. Handbook of olfaction and gustation. 2003:165–180. doi: 10.1016/B978-0-444-63855-7.00006-X. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci. 2011;5:69. doi: 10.3389/fnbeh.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckman FH, Freeman WJ. Correlations between unit firing and EEG in the rat olfactory system. Brain Res. 1990;528:238–244. doi: 10.1016/0006-8993(90)91663-2. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Ferry B, Oberling P, Jarrard LE, Di Scala G. Facilitation of conditioned odor aversion by entorhinal cortex lesions in the rat. Behavioral Neuroscience. 1996;110:443–450. doi: 10.1037//0735-7044.110.3.443. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd Edition. New York: Academic Press; 2008. [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. I. systems originating in the piriform cortex and adjacent areas. J Comp Neurol. 1978;178:711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Brandon MP. Linking cellular mechanisms to behavior: entorhinal persistent spiking and membrane potential oscillations may underlie path integration, grid cell firing, and episodic memory. Neural Plast. 2008;2008 doi: 10.1155/2008/658323. 658323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Herrero MT, Witter MP. Entorhinal cortex of the rat: cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus. 1997;7:146–183. doi: 10.1002/(SICI)1098-1063(1997)7:2<146::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA. Olfactory cortical adaptation facilitates detection of odors against background. Journal of Neurophysiology. 2006a;95:1888–1896. doi: 10.1152/jn.00812.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA. Olfactory cortical adaptation facilitates detection of odors against background. J Neurophysiol. 2006b;95:1888–1896. doi: 10.1152/jn.00812.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Freeman WJ. Bidirectional processing in the olfactory-limbic axis during olfactory behavior. Behav Neurosci. 1998;112:541–553. doi: 10.1037//0735-7044.112.3.541. [DOI] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17:697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Ketchum KL, Haberly LB. Membrane currents evoked by afferent fiber stimulation in rat piriform cortex. I. Current source-density analysis. J Neurophysiol. 1993;69:248–260. doi: 10.1152/jn.1993.69.1.248. [DOI] [PubMed] [Google Scholar]
- Litaudon P, Amat C, Bertrand B, Vigouroux M, Buonviso N. Piriform cortex functional heterogeneity revealed by cellular responses to odours. Eur J Neurosci. 2003;17:2457–2461. doi: 10.1046/j.1460-9568.2003.02654.x. [DOI] [PubMed] [Google Scholar]
- Lovitz AM, Sloan AM, Rennaker RL, Wilson DA. Complex mixture discrimination and the role of contaminants. Chemical Senses. 2012 doi: 10.1093/chemse/bjs006. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The laminar distribution of intracortical fibers originating in the olfactory cortex of the rat. J Comp Neurol. 1983;216:292–302. doi: 10.1002/cne.902160306. [DOI] [PubMed] [Google Scholar]
- Mayeaux DJ, Johnston RE. Discrimination of social odors and their locations: role of lateral entorhinal area. Physiol Behav. 2004;82:653–662. doi: 10.1016/j.physbeh.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Mouly AM, Di Scala G. Entorhinal cortex stimulation modulates amygdala and piriform cortex responses to olfactory bulb inputs in the rat. Neuroscience. 2006;137:1131–1141. doi: 10.1016/j.neuroscience.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Neville KR, Haberly LB. Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. J Neurophysiol. 2003;90:3921–3930. doi: 10.1152/jn.00475.2003. [DOI] [PubMed] [Google Scholar]
- Otto T, Schottler F, Staubli U, Eichenbaum H, Lynch G. Hippocampus and olfactory discrimination learning: effects of entorhinal cortex lesions on olfactory learning and memory in a successive-cue, go-no-go task. Behavioral Neuroscience. 1991;105:111–119. doi: 10.1037//0735-7044.105.1.111. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Alvarez P, Eichenbaum H. Neural correlates of social odor recognition and the representation of individual distinctive social odors within entorhinal cortex and ventral subiculum. Neuroscience. 2005;130:259–274. doi: 10.1016/j.neuroscience.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Input and output stations of the entorhinal cortex: superficial vs. deep layers or lateral vs. medial divisions? Brain Res Brain Res Rev. 2003;42:243–251. doi: 10.1016/s0165-0173(03)00175-9. [DOI] [PubMed] [Google Scholar]
- Sosulski DL, Lissitsyna Bloom M, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Ivy G, Lynch G. Hippocampal denervation causes rapid forgetting of olfactory information in rats. Proc Natl Acad Sci U S A. 1984;81:5885–5887. doi: 10.1073/pnas.81.18.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer's disease. Neural Plast. 2010;2010 doi: 10.1155/2010/108190. 108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA. Perception and the medial temporal lobe: evaluating the current evidence. Neuron. 2009;61:657–666. doi: 10.1016/j.neuron.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. Journal of Neurophysiology. 1998;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron. 2011;72:506–519. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth S, Ferry B, Di Scala G. Facilitation of olfactory recognition by lateral entorhinal cortex lesion in rats. Behavioural Brain Research. 1998a;91:49–59. doi: 10.1016/s0166-4328(97)00102-2. [DOI] [PubMed] [Google Scholar]
- Wirth S, Ferry B, Di Scala G. Facilitation of olfactory recognition by lateral entorhinal cortex lesion in rats. Behav Brain Res. 1998b;91:49–59. doi: 10.1016/s0166-4328(97)00102-2. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Fransen E, Hasselmo ME. mGluR-dependent persistent firing in entorhinal cortex layer III neurons. Eur J Neurosci. 2008;28:1116–1126. doi: 10.1111/j.1460-9568.2008.06409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]