Abstract
The activity of antimicrobial peptides has been shown to depend on the composition of the target cell membrane. The bacterial selectivity of most antimicrobial peptides has been attributed to the presence of abundant acidic phospholipids and the absence of cholesterol in bacterial membranes. The high amount of cholesterol present in eukaryotic cell membranes is thought to prevent peptide-induced membrane disruption by increasing the cohesion and stiffness of the lipid bilayer membrane. While the role of cholesterol on an AMP-induced membrane disrupting activity has been reported for simple, homogeneous lipids bilayers systems, it is not well understood for complex, heterogeneous lipid bilayers exhibiting phase separation (or “lipid rafts”). In this study, we show that cholesterol does not inhibit the disruption of raft-containing DOPC:DPPC model membranes by four different cationic antimicrobial peptides, MSI-78, MSI-594, MSI-367 and MSI-843 which permeabilize membranes. Conversely, the presence of cholesterol effectively inhibits the disruption of non-raft containing DOPC or DPPC lipid bilayers, even for antimicrobial peptides that do not show a clear preference between the ordered gel and disordered liquid-crystalline phases. Our results show that the peptide selectivity is not only dependent on the lipid phase but also on the presence of phase separation in heterogeneous lipid systems.
Keywords: antimicrobial peptide, membrane selectivity, raft domains, phase separation, MSI-78, MSI-843, MSI-367, MSI-594
Introduction
Antimicrobial peptides (AMPs) are small, highly cationic, amphipathic peptides known for their cell-selective membrane lytic activities.1–3 Most AMPs, like magainins or cecropins, preferentially act on bacterial cells,4–6 yet others like melittin and gramicidins have been shown to interact with both bacteria and eukaryotic cells.5, 7 Bacterial selectivity is believed to be linked largely to the ability of antimicrobial peptides to discriminate between different membrane types.8, 9 For the majority of these peptides, their cationic nature accounts for the selective disruption of bacterial membranes, since bacterial membranes contain significantly more acidic phospholipids than eukaryotic membranes (~10–70% of the total, depending on the species).10 Furthermore, the distribution of lipids is non-uniform in eukaryotic cells, with the acidic lipids largely concentrated in the inner leaflet in eukaryotic membranes.11 As such, studies considering the influence of lipids in the membrane targeting of antimicrobial peptides has largely focused on the role of negatively charged phospholipids. However, the action of these peptides is also dependent on membrane cholesterol levels, a component primarily found in eukaryotic membranes.12
In most homogeneous lipid systems, cholesterol is known to increase membrane cohesion and mechanical stiffness.13, 14 The presence of membrane-stabilizing cholesterol has been shown to protect human erythrocytes from attack by magainin 2.9, 15 Previous studies have also shown a protective effect of cholesterol on the membrane disrupting activity of other antimicrobial peptides such as pardaxin.16–19 From these studies, it has been inferred that cholesterol plays an important role in the selective targeting of AMPs to bacterial membranes over eukaryotic ones.8 However, both bacterial and eukaryotic cell membranes are actually complex mixtures of lipids whose physical properties vary non-linearly with the composition of the membrane. In particular, liquid ordered – liquid disordered (Lo-Ld) phase separation in eukaryotic membranes (i.e., the formation of “raft” domains) has been shown to play essential roles in the organization and activity of membrane proteins.20 Few studies have systematically looked at membrane disruption by AMPs in such systems. An exception is two studies by the Almeida group, which systematically studied the membrane permeabilizing activity of δ-lysin in raft-like palmitoyl-2-oleoylphosphatidylcholine/cholesterol/sphingomyelin (POPC/Chol/SM) mixtures.21, 22 These studies showed that membrane permeabilization by δ-lysin occurs exclusively in the Ld phase in membranes with Ld-Lo phase segregation and that the localization of δ-lysin to the Ld phase results in greater membrane disruption than would be expected in the absence of phase segregation.22 We generalize this important result to a diverse set of AMPs encompassing several membrane disruptive mechanisms, including AMPs that do not show a clear preference for either the gel or liquid crystalline phases. Our results further show that phase separation nullifies the effect of cholesterol against membrane disruption for all the AMPs tested. Importantly, we show that the formation of the Lo phase by cholesterol strongly inhibits membrane disruption even for AMPs that are active against the similarly ordered gel phase, implying the resistance to membrane disruption of the Lo phase by AMPs is not simply a result of increased acyl chain packing or bilayer thickness.
Materials and Methods
Materials
DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), DPPC (1,2-dipalmitoyol-sn-glycero-3-phosphocholine), and cholesterol were obtained from Avanti Polar Lipids Inc. (Alabaster, AL) and used without further purification or modification. Chloroform and methanol were purchased from Aldrich Chemical (Milwaukee, WI). Carboxyfluorescein (99%) was purchased from ACROS (Pittsburg, PA). All of the peptides were synthesized and donated by Genaera Corporation (Plymouth Meeting, PA).
Preparation of lipid vesicles
Stock solutions of DOPC (20 mg/mL), DPPC (20 mg/mL), and cholesterol (20 mg/mL) in chloroform were used to prepare a set of 16 samples with DOPC/DPPC/cholesterol molar ratios of 1/0/0, 80/0/20, 70/0/30, 60/0/40, 0/1/0, 0/80/20, 0/70/30, 0/60/40, 1/1/0, 40/40/20, 35/35/30, 30/30/40, 33/66/0, 26/53/20, 23/46/30, and 20/40/40. The concentration of total phospholipid (DOPC/DPPC) was held constant at an initial mixing concentration of 7 mM. The appropriate volumes of stock solution for each sample were mixed in a small, round-bottomed flask and the solvent was removed by evaporation over a gentle stream of dry nitrogen gas. Residual solvent was removed under vacuum overnight at room temperature. After the complete removal of solvents, the dry lipid films were hydrated at room temperature in the same small, round-bottomed flask with carboxyfluorescein dye at a concentration of 70 mM in 10 mM pH 7.5 sodium phosphate buffer without NaCl. The hydrated mixture was then carefully mixed by hand using a small glass rod and then transferred to a snap-cap centrifuge tube. To conclude the mixing process, samples were routinely subjected to five freeze-thaw cycles in which each mixture was frozen via liquid nitrogen submersion and then heated to 60 °C. The mixture was then kept at a constant 60 °C (well above the chain melting temperature of both lipids used) and was passed twenty-one times through a stainless steel extruder containing two nylon filters and a polycarbonate membrane containing 100 nm pores obtained from Fisher Scientific (Wayne, Mi) to produce a homogenous mixture of large unilamellar vesicles (LUVs). Non-encapsulated carboxyfluorescein was removed from the vesicle solution through size exclusion chromatography using a PD-10 column (Amersham Pharmacia Biotech, Uppsala, Sweden). LUV solution subsequently contained 10 mM sodium phosphate buffer and 100 mM NaCl at pH 7.5. This combination of buffers ensures that the osmotic strength is matched between the inside and outside of dye-filled LUVs, as determined by baseline leakage in the absence of peptide. Freshly prepared LUVs were used for each experiment.
Fluorescence experiments
Fluorescence readings were taken at an excitation wavelength of 493 nm and an emission wavelength of 518 nm. A baseline reading was taken on the solutions prior to the addition of peptide. Immediately after addition of the AMP, the fluorescence intensity was recorded for 900 seconds of interaction. The fluorescence signal given by the addition of peptide was then normalized by the addition of Triton X detergent, causing all vesicles present to release any remaining dye to obtain the total possible fluorescent signal.
Results and Discussion
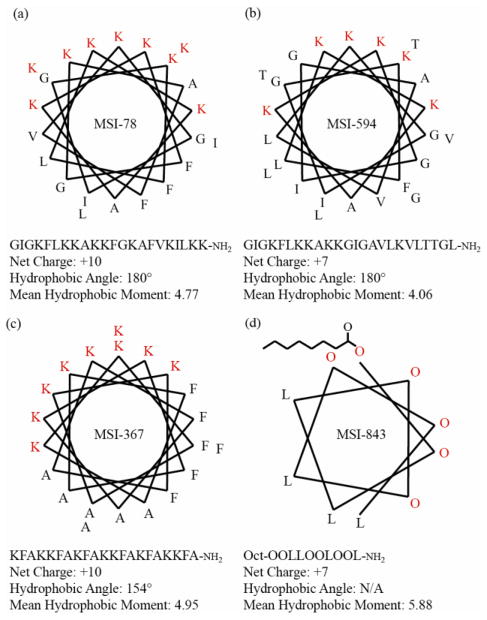
We have measured the effect of cholesterol on the membrane disrupting properties of four different antimicrobial peptides (MSI-78, MSI-594, MSI-843, and MSI-367) against both raft-containing membranes with liquid-disordered - liquid-ordered (Ld-Lo) phase separation (DOPC/DPPC/Chol) and non-raft membranes in the liquid crystalline (La) and gel phases (DOPC/Chol and DPPC/Chol). The phase boundaries of these systems have been extensively mapped at the temperature used in this study, 25 °C, by a number of groups.23–28 The amino acid sequences and other properties of the AMPs used are given in Fig. 1. The antimicrobial peptides chosen are highly potent against a broad-spectrum of both Gram-positive and Gram-negative bacteria and encompass a diverse set of membrane permeabilizing mechanisms and peptide-lipid interactions, 29–35 including MSI-78 (pexiganin), which was in Phase II clinical trials for the treatment of diabetic foot ulcers. 36–41
Figure 1.
Helical wheel projections, amino acid sequences, and physical parameters of (a) MSI-78, (b) MSI-594, (c) MSI-367, and (d) MSI-843. MSI-78 is an analog of Magainin-2, and MSI-594 is a hybrid of MSI-78 and melittin, a bee venom toxin.
Membrane disruption by AMPs can be selective for either the gel or liquid crystalline phase in the absence of cholesterol
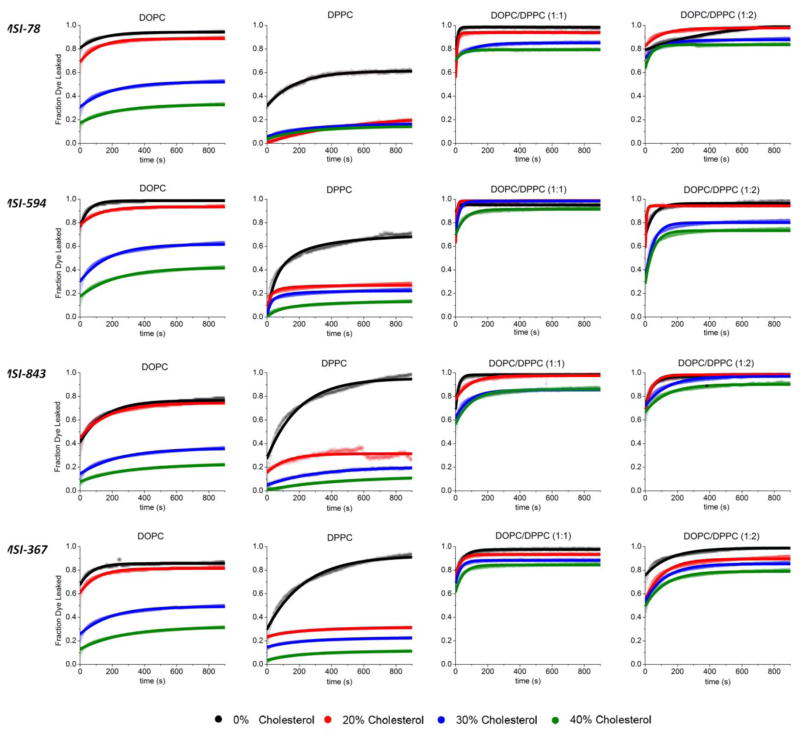
The release of the carboxyfluorescein dye from LUVs was measured as a function of time to test for differences in the extent of membrane disruption induced by MSI-78, MSI-594, MSI-843, and MSI-367 peptides in each of the 16 different lipid systems used in this study (Figs. 2 and 3). Carboxyfluorescein is self-quenched in intact LUVs at the high concentration (40 mM) used in the experiment. Disruption of the membrane by a peptide allows carboxyfluorescein to be released from LUVs, eliminating the self-quenching effect and therefore increasing the fluorescence.
Figure 2.
Fluorescence time curves for the release of encapsulated carboxyfluorescein dye from 100 nm LUVs upon incubation with the indicated antimicrobial peptide. Each sample was maintained at a peptide/lipid ratio of 1/1000 and a temperature of 25°C. Dye release is essentially complete after 900 seconds for all samples.
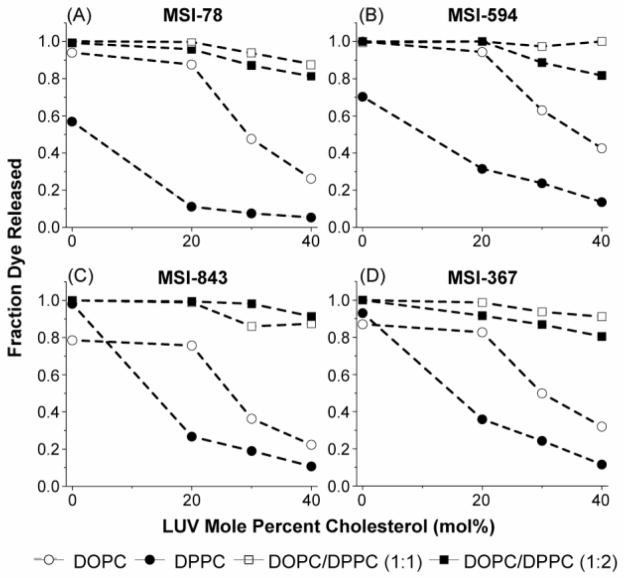
Figure 3.
Fraction of dye released after 900 seconds from model LUVs (DOPC open circles, DPPC filled circles, DOPC/DPPC (1/1) open squares; DOPC/DPPC (1/2) filled squares) as a function of the concentration of cholesterol present in the membrane when incubated with MSI-78, MSI-594, MSI-843, and MSI-367.
Since cholesterol exerts a strong ordering effect on the acyl chain order of the bilayer,42 we began by examining the degree of membrane disruption by each AMP in LUVs that are either in the ordered gel phase (DPPC LUVs) or the more disordered liquid crystalline Lα phase (DOPC LUVs) (Fig. 3, filled and open circles respectively).23 Three of the four antimicrobial peptides used in this investigation clearly favor a specific lipid phase. Both MSI-78 and MSI-594 have a marked selectivity for disordered, liquid crystalline (Lα) phase lipids, as shown by the greater amounts of dye released in DOPC LUVs compared to DPPC LUVs (open compared to filled circles in Fig. 3A and B). This observation is supported by previous investigations showing that MSI-78 induces significant changes in bilayer structure that are indicative of toroidal pore formation.30, 39, 43–45 We attribute a similar mechanism to MSI 594 because MSI-594 is a synthetic hybrid of MSI-78 (residues 1–11) and the bee venom toxin melittin (residues 12–24) and also shows a similar random coil to alpha-helix structural transition as MSI-78.46 Toroidal pore formation requires partial insertion of the peptide into the bilayer.31–33 Such peptide insertion is dependent upon lipid order and packing, as demonstrated by the antimicrobial peptide protegrin-1.47 The greater degree of membrane disruption observed for MSI-78 and MSI-843 in liquid crystalline samples therefore matches expectations based on the energetics of peptide insertion, as peptide insertion is energetically more difficult in gel phase lipids.
Membrane disruption by MSI-843, however, is more favorable in gel phase lipids, as indicated by the greater amounts of dye released in DPPC liposomes compared to DOPC liposomes in the absence of cholesterol (filled circles compared to open circles in Fig. 3C). MSI-843 is a lipopeptide consisting of a single fully saturated chain of octanoic acid attached to a short helical stretch of the nonstandard amino acid ornithine.29 Observing that the MSI-843 lipopeptide prefers the more hydrophobic, ordered phase is not surprising, as this behavior is common to other lipidated peptides.48 MSI-367 does not appear to have a strong dependence on a specific phase, yet it has shown highly potent activity for each single-phase system. (Fig. 3D) This behavior could be because the peptide prefers to remain at the lipid-water interface,35 which is similar in both phases.
Cholesterol inhibits membrane disruption by AMPs in non-raft membranes
We next examined the effect of cholesterol on membrane disruption by AMPS in non-raft membranes (DOPC/Chol and DPPC/Chol, open and filled circles in Fig. 3, respectively). For all the AMPs tested, AMP induced membrane disruption in non-raft LUVs decreases as the cholesterol content is increased. However, the concentration dependence of the effect is different for DOPC and DPPC liposomes. For DOPC membranes, the effect is nonlinear with the cholesterol concentration, with a sharp decrease in membrane disruption occurring when the cholesterol concentration was increased above 20%. DOPC LUVs incorporating 20% cholesterol are only slightly more resistant to disruption than DOPC LUVs without cholesterol (< 5% difference for each AMP) (Fig. 3 open circles). Only as the cholesterol content is increased beyond the 20% threshold is a sharp reduction in membrane disruption observed for all the AMPs tested (~45 % at 30% cholesterol). This finding neatly corresponds with the known phase properties of DOPC cholesterol mixtures. Below a concentration threshold of 20% cholesterol, DOPC/Chol membranes exist as a mixture of the Lo and liquid crystalline (Lα) phases.23 Above the 20% concentration threshold, DOPC/Chol liposomes exist purely in the Lo phase.23 The existence of a sharp reduction in membrane disruption at 20% cholesterol suggests that while AMPs are excluded from the Lo phase, they are free to attack Lα domains on the remainder of the membrane.22 Membrane disruption in DPPC liposomes, on the other hand, is strongly inhibited even by the incorporation of 20% cholesterol (Fig. 3, filled circles). This finding is also consistent with the known phase properties of DPPC/cholesterol membranes, which are almost entirely in the Lo phase at 20% cholesterol at 25 °C.22, 42
Resistance of the Lo phase to membrane disruption is observed even in the two AMPs (MSI 843 and MSI 367) that do not show a clear preference for the less tightly packed liquid crystalline Lα phase over the rigid gel phase (Fig. 3C and D). The properties of the Lo phase are in most respects intermediate between the liquid crystalline and gel phases, including bilayer thickness, acyl chain ordering, viscosity, and elastic modulus.14, 49, 50 Based on these findings, it might be expected that membrane disruption in Lo phases should also be intermediate between the values found for the liquid crystalline Lα and gel phases. However, Fig. 3 suggests this is clearly not the case. Membrane disruption for all the AMPs tested is clearly lower for the Lo phase than either the gel or liquid crystalline Lα phases. However, not all properties of the Lo phase are intermediate between the gel and liquid crystalline phases. Hydration in the interfacial region of the membrane is significantly lower in the Lo phase compared to the gel phase,51 most likely due to the formation of hydrogen bonds from the 3-OH group of cholesterol to the Sn2 and phosphate group of lipids.52 This result suggests that cholesterol can compete with the peptide for electrostatic and hydrogen binding to the Sn2 and phosphate groups of the lipids.
Raft-domain containing LUVs are disrupted by AMPs irrespective of the presence of cholesterol
In non-raft membranes, cholesterol strongly inhibits membrane disruption at moderate concentrations for all the AMPs tested. However, this is not true for raft type mixtures of lipids in which phase separation is expected. We observed high fractions of dye release (80–100 %) for all AMPs in both DOPC/DPPC (1/1) and DOPC/DPPC (1/2) systems when cholesterol was incorporated between 20 and 40 mole % (Fig. 3, open and filled squares), contrasting with the strongly attenuated membrane disruption in non-raft LUVs in this concentration range (Fig. 3, open and filled circles). Membrane disruption by AMPs in phase separated lipid systems does not decrease significantly with increasing membrane cholesterol content as it does for single-phase lipid systems. Instead, only a slight decrease is observed as the cholesterol concentration is increased from 20–40% (5–15% change as opposed to 25–90% change). This finding is consistent with preferential targeting of AMP to Lα domains in systems with Lo and Lα coexistence (Fig. 4). We cannot exclude an additional effect from line tension at the Lo-Lα interface based on this data, yet we believe that the relatively minimal hydrophobic mismatch afforded by our lipid systems would not be supportive of such a model.53, 54
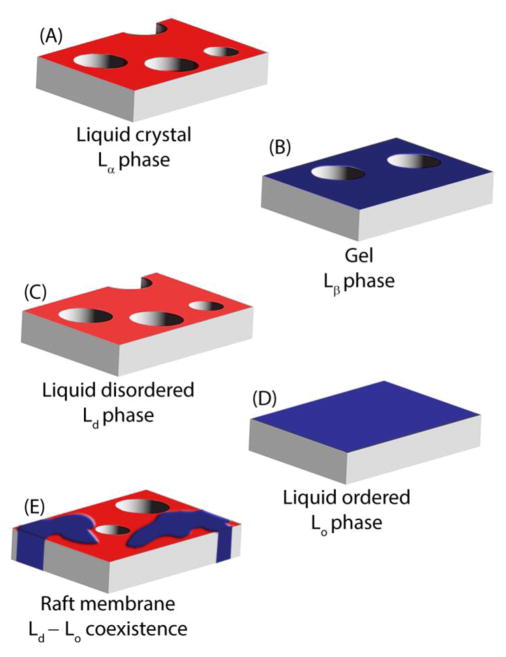
Figure 4.
Cartoon showing membrane disruption in liquid crystalline (Lα) (A), gel (B), liquid disordered (Ld) (C), and raft membranes with liquid disordered-liquid ordered coexistence (E), but not in membranes in the liquid ordered phase only (D).
Conclusion
We have shown here the importance of lipid heterogeneity and phase on antimicrobial peptide selectivity. Most importantly, we show that the addition of cholesterol to membranes that have phase separation does not greatly inhibit peptide activity to the extent that it does for homogeneous lipid systems of either ordered or disordered lipids. We conclude that cholesterol’s protective, membrane stabilizing effect does not occur to an appreciable level in lipid systems containing raft domains. These results not only provide insight into the processes by which MSI-78, MSI-594, MSI-843, and MSI-367 interact with lipid membranes, but they also broaden the understanding of the interactions involved in general antimicrobial peptide targeting. This information may also prove useful for the design of heterogeneous model membranes for the use of various peptide-lipid interactions.
Highlights.
Effect of cholesterol on antimicrobial peptide induced membrane disruption in different lipid phases was tested.
Cholesterol had no effect on the AMP-induced membrane disruption in liquid disordered phase.
Formation of liquid ordered phase by cholesterol strongly inhibited membrane disruption by an AMP.
Cholesterol had no effect on the disruption of raft membranes with liquid ordered-liquid disordered coexistence.
Acknowledgments
This study was partly supported by research funds from NIH (GM084018 and GM095640 to A.R.).
Abbreviations
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DPPC
1,2-dipalmitoyol-sn-glycero-3-phosphocholine
- POPC
1-palmitoyl-2-oleoylphosphatidylcholine
- SM
sphingomyelin
- Chol
cholesterol
- AMP
antimicrobial peptide
- LUV
large unilamellar vesicle
- Ld
liquid-disordered
- Lo
liquid-ordered
- Lα
liquid-crystalline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 2.Bechinger B. Insights into the mechanisms of action of host defence peptides from biophysical and structural investigations. J Pep Sci. 2011;17:306–314. doi: 10.1002/psc.1343. [DOI] [PubMed] [Google Scholar]
- 3.Pius J, Morrow MR, Booth V. H-2 solid-state nuclear magnetic resonance investigation of whole Escherichia coli interacting with antimicrobial peptide MSI-78. Biochemistry. 2012;51:118–125. doi: 10.1021/bi201569t. [DOI] [PubMed] [Google Scholar]
- 4.Chen HC, Brown JH, Morell JL, Huang CM. Synthetic magainin analogs with improved antimicrobial activity. FEBS Lett. 1988;236:462–466. doi: 10.1016/0014-5793(88)80077-2. [DOI] [PubMed] [Google Scholar]
- 5.Steiner H, Hultmark D, Engstrom A, Bennich H, Boman HG. Sequence and specificity of 2 anti-bacterial proteins involved in insect immunity. Nature. 1981;292:246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 6.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin - Isolation, characterization of 2 active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsu T, Kuroko M, Morikawa T, Sanchika K, Fujita Y, Yamamura H, Uda M. Mechanism of membrane damage induced by the amphipathic peptides gramicidin-S and melittin. Biochim Biophys Acta. 1989;983:135–141. doi: 10.1016/0005-2736(89)90226-5. [DOI] [PubMed] [Google Scholar]
- 8.Glukhov E, Stark M, Burrows LL, Deber CM. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J Biol Chem. 2005;280:33960–33967. doi: 10.1074/jbc.M507042200. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzaki K, Sugishita K, Fujii N, Miyajima K. Molecular-basis for membrane selectivity of an antimicrobial peptide, magainin-2. Biochemistry. 1995;34:3423–3429. doi: 10.1021/bi00010a034. [DOI] [PubMed] [Google Scholar]
- 10.Epand RF, Savage PB, Epand RM. Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins) Biochim Biophys Acta. 2007;1768:2500–2509. doi: 10.1016/j.bbamem.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 11.Riedl S, Zweytick D, Lohner K. Membrane-active host defense peptides - Challenges and perspectives for the development of novel anticancer drugs. Chem Phys Lipids. 2011;164:766–781. doi: 10.1016/j.chemphyslip.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta. 1999;1462:1–10. doi: 10.1016/s0005-2736(99)00197-2. [DOI] [PubMed] [Google Scholar]
- 13.Evans EA, Waugh R. Mechano-Chemistry of Closed, Vesicular Membrane Systems. J Colloid Interface Sci. 1977;60:286–298. [Google Scholar]
- 14.Henriksen J, Rowat AC, Brief E, Hsueh YW, Thewalt JL, Zuckermann MJ, Ipsen JH. Universal behavior of membranes with sterols. Biophys J. 2006;90:1639–1649. doi: 10.1529/biophysj.105.067652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuzaki K, Murase O, Fujii N, Miyajima K. Translocation of a channel-forming antimicrobial peptide, magainin-2, across lipid bilayers by forming a pore. Biochemistry. 1995;34:6521–6526. doi: 10.1021/bi00019a033. [DOI] [PubMed] [Google Scholar]
- 16.Epand RF, Ramamoorthy A, Epand RM. Membrane lipid composition and the interaction of pardaxin: The role of cholesterol. Prot Pept Lett. 2006;3:1–5. [PubMed] [Google Scholar]
- 17.Ramamoorthy A, Lee DK, Narasimhaswamy T, Nanga RPR. Cholesterol reduces pardaxin’s dynamics-a barrel-stave mechanism of membrane disruption investigated by solid-state NMR. Biochim Biophys Acta. 2010;1798:223–227. doi: 10.1016/j.bbamem.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wildman KAH, Lee DK, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 19.Hallock KJ, Lee DK, Omnaas J, Mosberg HI, Ramamoorthy A. Membrane composition determines pardaxin’s mechanism of lipid bilayer disruption. Biophys J. 2002;83:1004–1013. doi: 10.1016/S0006-3495(02)75226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 21.Pokorny A, Yandek LE, Elegbede AI, Hinderliter A, Almeida PFF. Temperature and composition dependence of the interaction of δ-lysin with ternary mixtures of sphingomyelin/cholesterol/POPC. Biophys J. 2006;91:2184–2197. doi: 10.1529/biophysj.106.085027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pokorny A, Almeida PFF. Permeabilization of raft-containing lipid vesicles by δ-lysin: A mechanism for cell sensitivity to cytotoxic peptides. Biochemistry. 2005;44:9538–9544. doi: 10.1021/bi0506371. [DOI] [PubMed] [Google Scholar]
- 23.Marsh D. Liquid-ordered phases induced by cholesterol: A compendium of binary phase diagrams. Biochim Biophys Acta. 2010;1798:688–699. doi: 10.1016/j.bbamem.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 24.Davis JH, Clair JJ, Juhasz J. Phase equilibria in DOPC/DPPC-d(62)/cholesterol mixtures. Biophys J. 2009;96:521–539. doi: 10.1016/j.bpj.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veatch SL, Keller SL. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys J. 2003;85:3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veatch SL, Polozov IV, Gawrisch K, Keller SL. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys J. 2004;86:2910–2922. doi: 10.1016/S0006-3495(04)74342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang TH, Lee CWB, Dasgupta SK, Blume A, Griffin RG. A C-13 and H-2 nuclear-magnetic-resonance study of phosphatidylcholine cholesterol interactions - Characterization of liquid-gel phases. Biochemistry. 1993;32:13277–13287. doi: 10.1021/bi00211a041. [DOI] [PubMed] [Google Scholar]
- 28.Veatch SL, Soubias O, Keller SL, Gawrisch K. Critical fluctuations in domain-forming lipid mixtures. Proc Natl Acad Sci U S A. 2007;104:17650–17655. doi: 10.1073/pnas.0703513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thennarasu S, Lee DK, Tan A, Kari UP, Ramamoorthy A. Antimicrobial activity and membrane selective interactions of a synthetic lipopeptide MSI-843. Biochim Biophys Acta. 2005;1711:49–58. doi: 10.1016/j.bbamem.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallock KJ, Lee DK, Ramamoorthy A. MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain. Biophys J. 2003;84:3052–3060. doi: 10.1016/S0006-3495(03)70031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramamoorthy A, Thennarasu S, Lee DK, Tan AM, Maloy L. Solid-state NMR investigation of the membrane-disrupting mechanism of antimicrobial peptides MSI-78 and MSI-594 derived from magainin 2 and melittin. Biophys J. 2006;91:206–216. doi: 10.1529/biophysj.105.073890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porcelli F, Buck-Koehntop BA, Thennarasu S, Ramamoorthy A, Veglia G. Structures of the dimeric and monomeric variants of magainin antimicrobial peptides (MSI-78 and MSI-594) in micelles and bilayers, determined by NMR spectroscopy. Biochemistry. 2006;45:5793–5799. doi: 10.1021/bi0601813. [DOI] [PubMed] [Google Scholar]
- 33.Domadia PN, Bhunia A, Ramamoorthy A, Bhattacharjya S. Structure, interactions, and antibacterial activities of MSI-594 derived mutant peptide MSI-594F5A in lipopolysaccharide micelles: Role of the helical hairpin conformation in outer-membrane permeabilization. J Am Chem Soc. 2010;132:18417–18428. doi: 10.1021/ja1083255. [DOI] [PubMed] [Google Scholar]
- 34.Epand RF, Maloy WL, Ramamoorthy A, Epand RM. Probing the “charge cluster mechanism” in amphipathic helical cationic antimicrobial peptides. Biochemistry. 2010;49:4076–4084. doi: 10.1021/bi100378m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thennarasu S, Huang R, Lee DK, Yang P, Maloy L, Chen Z, Ramamoorthy A. Limiting an antimicrobial peptide to the lipid-water interface enhances its bacterial membrane selectivity: A case study of MSI-367. Biochemistry. 2010;49:10595–10605. doi: 10.1021/bi101394r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipsky BA, Holroyd KJ, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: A randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis. 2008;47:1537–1545. doi: 10.1086/593185. [DOI] [PubMed] [Google Scholar]
- 37.Lamb HM, Wiseman LR. Pexiganan acetate. Drugs. 1998;56:1047–1052. doi: 10.2165/00003495-199856060-00011. [DOI] [PubMed] [Google Scholar]
- 38.Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, Claxton K, Bell-Syer SEM, Jude E, Dowson C, Gadsby R, O’Hare P, Powell J. A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers. Health Technol Assess. 2006;10:1–221. doi: 10.3310/hta10120. [DOI] [PubMed] [Google Scholar]
- 39.Gottler LM, Ramamoorthy A. Structure, membrane orientation, mechanism, and function of pexiganan - A highly potent antimicrobial peptide designed from magainin. Biochim Biophys Acta. 2009;1788:1680–1686. doi: 10.1016/j.bbamem.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giacometti A, Cirioni O, Kamysz W, D’Amato G, Silvestri C, Licci A, Nadolski P, Riva A, Lukasiak J, Scalise G. In vitro activity of MSI-78 alone and in combination with antibiotics against bacteria responsible for bloodstream infections in neutropenic patients. Int J Antimicrob Agents. 2005;26:235–240. doi: 10.1016/j.ijantimicag.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Giacometti A, Ghiselli R, Cirioni O, Mocchegiani F, D’Amato G, Orlando F, Sisti V, Kamysz W, Silvestri C, Naldoski P, Lukasiak J, Saba V, Scalise G. Therapeutic efficacy of the magainin analogue MSI-78 in different intra-abdominal sepsis rat models. J Antimicrob Chemother. 2004;54:654–660. doi: 10.1093/jac/dkh390. [DOI] [PubMed] [Google Scholar]
- 42.Vist MR, Davis JH. Phase-equilibria of cholesterol dipalmitoylphosphatidylcholine mixtures - H-2 nuclear magnetic-resonance and differential scanning calorimetry. Biochemistry. 1990;29:451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- 43.Mecke A, Lee DK, Ramamoorthy A, Orr BG, Holl MMB. Membrane thinning due to antimicrobial peptide binding: An atomic force microscopy study of MSI-78 in lipid bilayers. Biophys J. 2005;89:4043–4050. doi: 10.1529/biophysj.105.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto K, Vivekanandan S, Ramamoorthy A. Fast NMR data acquisition from bicelles containing a membrane-associated peptide at natural-abundance. J Phys Chem B. 2011;115:12448–12455. doi: 10.1021/jp2076098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang P, Ramamoorthy A, Chen Z. Membrane orientation of MSI-78 measured by sum frequency generation vibrational spectroscopy. Langmuir. 2011;27:7760–7767. doi: 10.1021/la201048t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhunia A, Ramamoorthy A, Bhattacharjya S. Helical hairpin structure of a potent antimicrobial peptide MSI-594 in lipopolysaccharide micelles by NMR spectroscopy. Chem-Eur J. 2009;15:2036–2040. doi: 10.1002/chem.200802635. [DOI] [PubMed] [Google Scholar]
- 47.Ishitsuka Y, Pham DS, Waring AJ, Lehrer RI, Lee KYC. Insertion selectivity of antimicrobial peptide protegrin-1 into lipid monolayers: Effect of head group electrostatics and tail group packing. Biochim Biophys Acta. 2006;1758:1450–1460. doi: 10.1016/j.bbamem.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Wang TY, Leventis R, Silvius JR. Partitioning of lipidated peptide sequences into liquid-ordered lipid domains in model and biological membranes. Biochemistry. 2001;40:13031–13040. doi: 10.1021/bi0112311. [DOI] [PubMed] [Google Scholar]
- 49.Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 50.McMullen TPW, Lewis RNAH, McElhaney RN. Cholesterol-phospholipid interactions, the liquid-ordered phase and lipid rafts in model and biological membranes. Curr Opin Colloid Interface Sci. 2004;8:459–468. [Google Scholar]
- 51.M’Baye G, Mely Y, Duportail G, Klymchenko AS. Liquid ordered and gel phases of lipid bilayers: Fluorescent probes reveal close fluidity but different hydration. Biophys J. 2008;95:1217–1225. doi: 10.1529/biophysj.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sankaram MB, Thompson TE. Cholesterol-induced fluid-phase immiscibility in membranes. Proc Natl Acad Sci U S A. 1991;88:8686–8690. doi: 10.1073/pnas.88.19.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honerkamp-Smith AR, Cicuta P, Collins MD, Veatch SL, den Nijs M, Schick M, Keller SL. Line tensions, correlation lengths, and critical exponents in lipid membranes near critical points. Biophys J. 2008;95:236–246. doi: 10.1529/biophysj.107.128421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia-Saez AJ, Chiantia S, Schwille P. Effect of line tension on the lateral organization of lipid membranes. J Biol Chem. 2007;282 doi: 10.1074/jbc.M706162200. [DOI] [PubMed] [Google Scholar]