Abstract
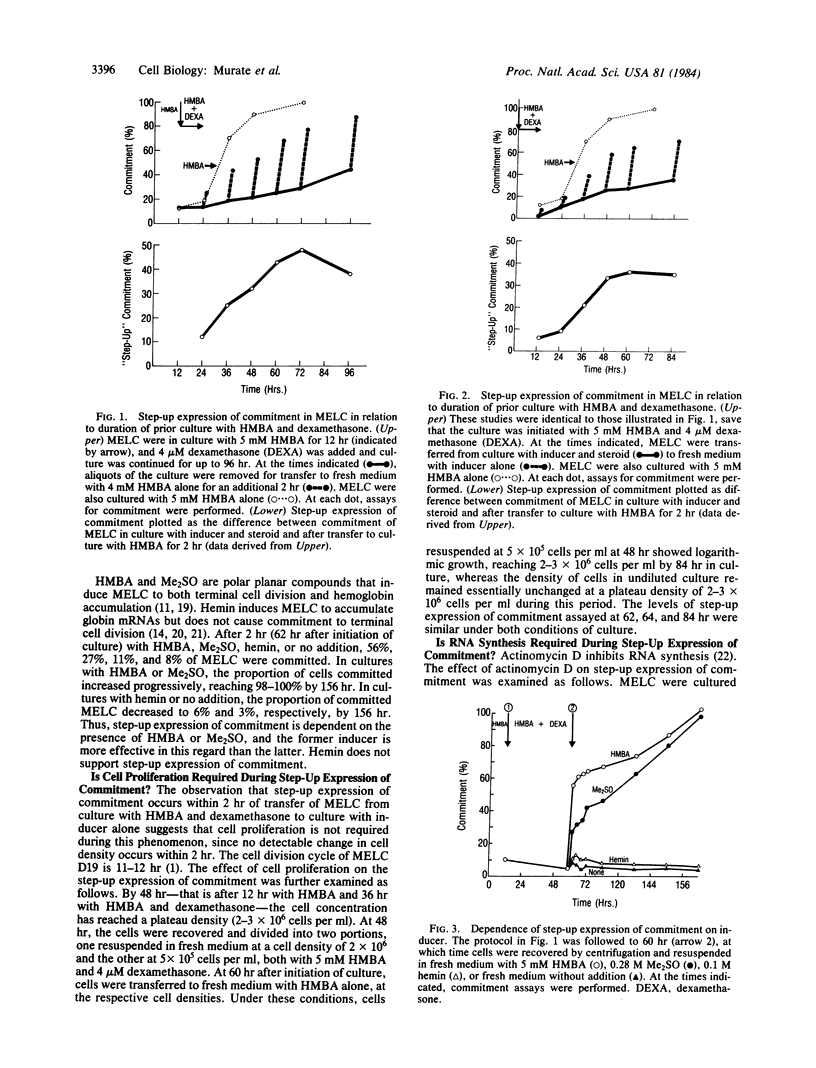
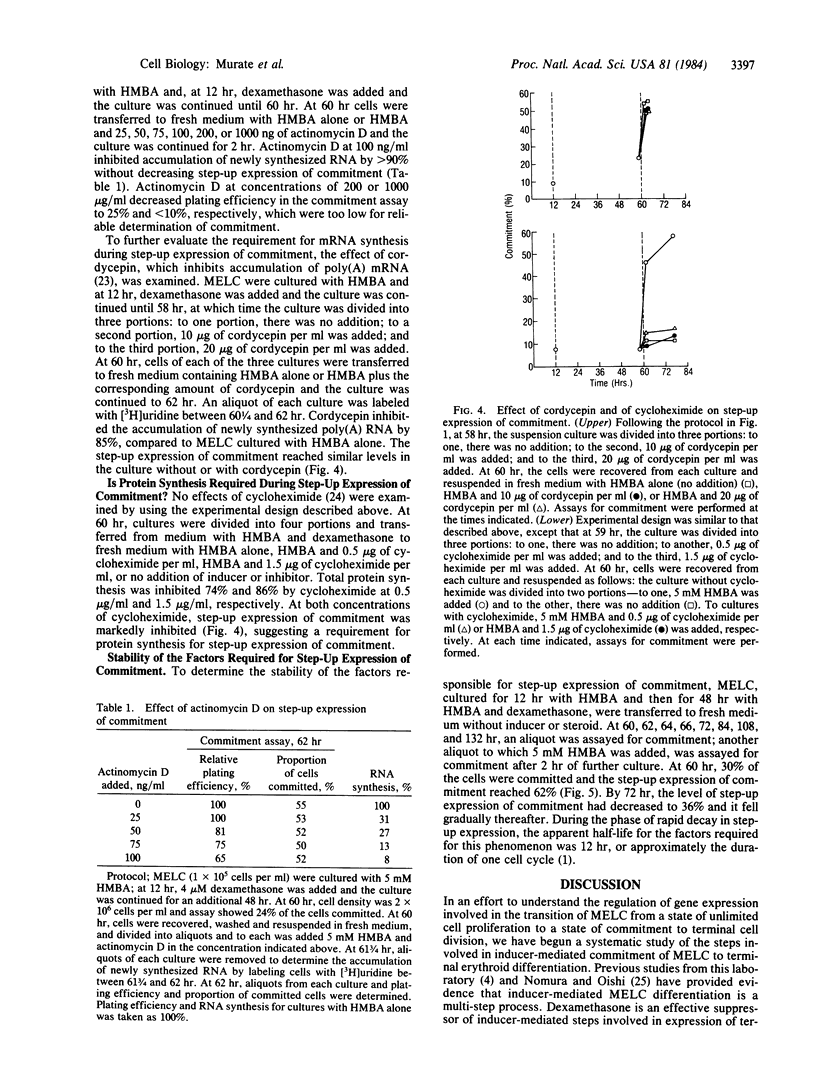
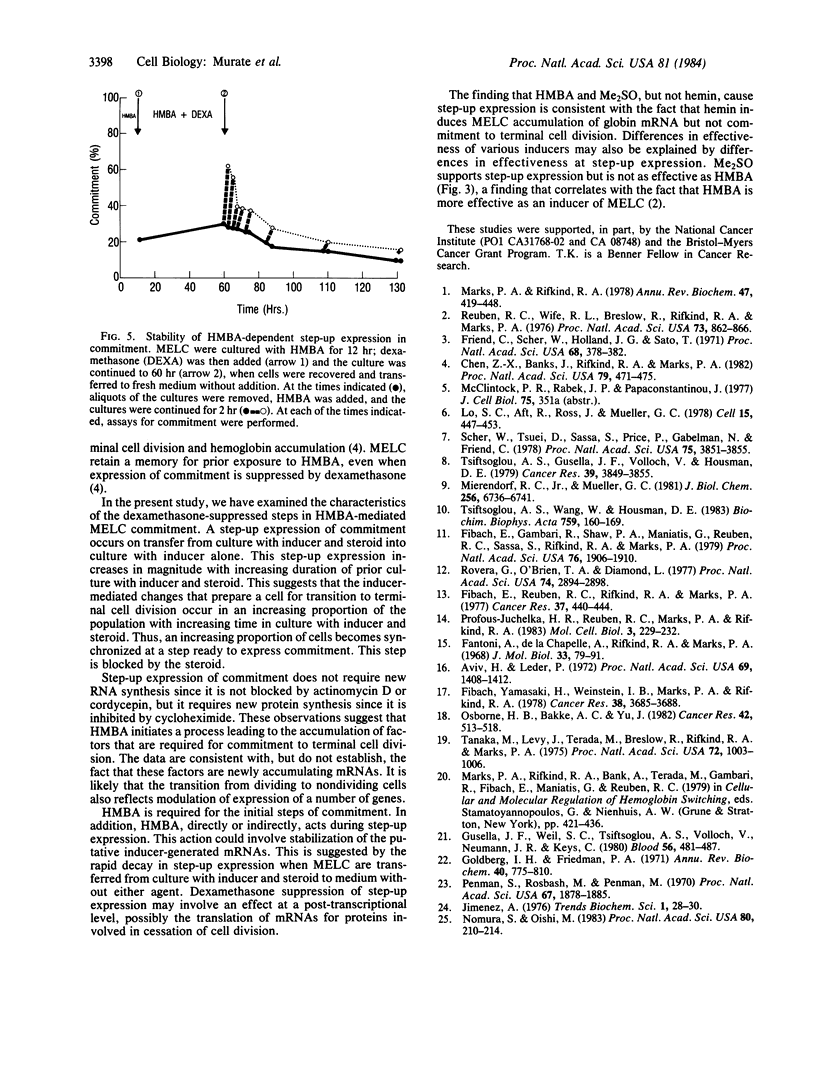
Murine erythroleukemia cells (MELC) are transformed cells that can be induced to differentiate by a variety of agents, such as hexamethylenebisacetamide (HMBA) and dimethyl sulfoxide. Dexamethasone suppresses HMBA-mediated MELC differentiation, but MELC retain a memory for their exposure to HMBA since, on transfer from culture with HMBA and dexamethasone to medium without additions, a portion of the cells express characteristics of terminal differentiation. This study characterizes the steroid suppressed steps in the multi-step process of inducer-mediated MELC terminal differentiation. MELC in culture with HMBA and dexamethasone show low levels of commitment to terminal cell division; upon transfer to culture with inducer alone there is a rapid increase in the proportion of committed cells. The magnitude of this rapid or "step-up" expression of commitment increased with the length of prior culture with inducer and steroid. This step-up expression is not inhibited by actinomycin D or cordycepin but is blocked by cycloheximide. HMBA is required for step-up expression of commitment. In the absence of inducer, there is a rapid decay in the capacity for step-up expression. Thus, HMBA initiates a series of changes leading to the accumulation of factors--which may be mRNAs--whose expression is blocked by dexamethasone. Hemin, which induces MELC accumulation of globin mRNA but not commitment to terminal cell division, cannot, as does HMBA or dimethyl sulfoxide, cause step-up expression of commitment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Banks J., Rifkind R. A., Marks P. A. Inducer-mediated commitment of murine erythroleukemia cells to differentiation: a multistep process. Proc Natl Acad Sci U S A. 1982 Jan;79(2):471–475. doi: 10.1073/pnas.79.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantoni A., De la Chapelle A., Rifkind R. A., Marks P. A. Erythroid cell-development in fetal mice: synthetic capacity for different proteins. J Mol Biol. 1968 Apr 14;33(1):79–91. doi: 10.1016/0022-2836(68)90282-9. [DOI] [PubMed] [Google Scholar]
- Fibach E., Gambari R., Shaw P. A., Maniatis G., Reuben R. C., Sassa S., Rifkind R. A., Marks P. A. Tumor promoter-mediated inhibition of cell differentiation: suppression of the expression of erythroid functions in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1906–1910. doi: 10.1073/pnas.76.4.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibach E., Reuben R. C., Rifkind R. A., Marks P. A. Effect of hexamethylene bisacetamide on the commitment to differentiation of murine erythroleukemia cells. Cancer Res. 1977 Feb;37(2):440–444. [PubMed] [Google Scholar]
- Fibach E., Yamasaki H., Weinstein I. B., Marks P. A., Rifkind R. A. Heterogeneity of murine erythroleukemia cells with respect to tumor promoter-mediated inhibition of cell differentiation. Cancer Res. 1978 Nov;38(11 Pt 1):3685–3688. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I. H., Friedman P. A. Antibiotics and nucleic acids. Annu Rev Biochem. 1971;40:775–810. doi: 10.1146/annurev.bi.40.070171.004015. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Weil S. C., Tsiftsoglou A. S., Volloch V., Neumann J. R., Keys C., Housman D. E. Hemin does not cause commitment of murine erythroleukemia (MEL) cells to terminal differentiation. Blood. 1980 Sep;56(3):481–487. [PubMed] [Google Scholar]
- Lo S. C., Aft R., Ross J., Mueller G. C. Control of globin gene expression by steroid hormones in differentiating Friend leukemia cells. Cell. 1978 Oct;15(2):447–453. doi: 10.1016/0092-8674(78)90014-4. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Mierendorf R. C., Jr, Mueller G. C. Role of dexamethasone in globin gene expression in differentiating Friend cells. J Biol Chem. 1981 Jul 10;256(13):6736–6741. [PubMed] [Google Scholar]
- Nomura S., Oishi M. Indirect induction of erythroid differentiation in mouse Friend cells: evidence for two intracellular reactions involved in the differentiation. Proc Natl Acad Sci U S A. 1983 Jan;80(1):210–214. doi: 10.1073/pnas.80.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne H. B., Bakke A. C., Yu J. Effect of dexamethasone on hexamethylene bisacetamide-induced Friend cell erythrodifferentiation. Cancer Res. 1982 Feb;42(2):513–518. [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profous-Juchelka H. R., Reuben R. C., Marks P. A., Rifkind R. A. Transcriptional and post-transcriptional regulation of globin gene accumulation in murine erythroleukemia cells. Mol Cell Biol. 1983 Feb;3(2):229–232. doi: 10.1128/mcb.3.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R. C., Wife R. L., Breslow R., Rifkind R. A., Marks P. A. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):862–866. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Tumor promoters inhibit spontaneous differentiation of Friend erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2894–2898. doi: 10.1073/pnas.74.7.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher W., Tsuei D., Sassa S., Price P., Gabelman N., Friend C. Inhibition of dimethyl sulfoxide-stimulated Friend cell erythrodifferentiation by hydrocortisone and other steroids. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3851–3855. doi: 10.1073/pnas.75.8.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Levy J., Terada M., Breslow R., Rifkind R. A., Marks P. A. Induction of erythroid differentiation in murine virus infected eythroleukemia cells by highly polar compounds. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1003–1006. doi: 10.1073/pnas.72.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiftsoglou A. S., Gusella J. F., Volloch V., Housman D. E. Inhibition by dexamethasone of commitment to erythroid differentiation in murine erythroleukemia cells. Cancer Res. 1979 Oct;39(10):3849–3855. [PubMed] [Google Scholar]
- Tsiftsoglou A. S., Wong W., Housman D. E. Dexamethasone-sensitive and -insensitive responses during in vitro differentiation of Friend erythroleukemia cells. Biochim Biophys Acta. 1983 Sep 13;759(3):160–169. doi: 10.1016/0304-4165(83)90308-2. [DOI] [PubMed] [Google Scholar]



