Abstract
Morphine, a potent narcotic analgesic used for the treatment of acute and chronic pain, was chemically incorporated into a poly(anhydride-ester) backbone. The polymer termed “PolyMorphine”, was designed to degrade hydrolytically releasing morphine in a controlled manner to ultimately provide analgesia for an extended time period. PolyMorphine was synthesized via melt-condensation polymerization and its structure was characterized using proton and carbon nuclear magnetic resonance spectroscopies, and infrared spectroscopy. The weight-average molecular weight and the thermal properties were determined. The hydrolytic degradation pathway of the polymer was determined by in vitro studies, showing that free morphine is released. In vitro cytocompatibility studies demonstrated that PolyMorphine is non-cytotoxic towards fibroblasts. In vivo studies using mice showed that PolyMorphine provides analgesia for 3 days, 20 times the analgesic window of free morphine. The animals retained full responsiveness to morphine after being subjected to an acute morphine challenge.
Keywords: morphine, biodegradable, polymer, extended release, pain treatment, prodrug
1. Introduction
Morphine is a potent narcotic analgesic used for the treatment of acute and chronic pain, providing reliable analgesia.[1–6] However, morphine has a half-life in plasma of 2–4 h, requiring repeated administration to maintain the drug at therapeutic levels for an extended time period.[5–7] Repeated administration affects patient comfort because the daily activities of the patient will be interrupted in order to take the medication, which can lead to low compliance.[6–9] In addition, morphine use is accompanied by the development of tolerance and dependence, leading to an increase in dosing (i.e., amount and frequency).[1, 10] Other side effects that can result from morphine use are respiratory depression, somnolence, and gastrointestinal effects (e.g., nausea, vomiting, and constipation).[4, 5]
Controlled-release morphine formulations can prolong the analgesic effect of the drug and prevent accidental withdrawals due to missed doses.[4, 7] In recent years, the formulation of morphine delivery systems for controlled-release has increased. Various delivery systems that use enteral and parenteral administration are commercially available. Among the different administration routes, enteral is the most frequently used. Among commercially available morphine delivery systems (tablets or capsules) are Kadian®,[6, 10] Avinza®,[2, 5] and MS Contin®[7] that can release morphine for 12–24 h. Even though these tablets and capsules are successful at maintaining long-term benefits of the drug without dose escalation, these tablets and capsules are also sensitive to physical alterations that affect their release mechanism.[10, 11] When the tablet or capsule is crushed, chewed, or dissolved it increases the risk of administration of a fatal dose.[11] Because these formulations contain a large dose that can be easily separated (by crushing or breaking the tablet/capsule), they also increase the potential for recreational use.[6]
Other formulations have been extensively explored including lipid-based carriers,[9, 12–14] drug encapsulation within polymers,[15–18] and polymer-drug complexes.[19–21] Previously, morphine was chemically incorporated into a polyurethane backbone (as a pendant group); however, polyurethanes are resistant to biodegradation under physiological conditions and are of limited biological potential.[22] The major drawbacks of these formulations are low drug loading and/or rapid drug release, as usually evidenced by a burst release.
The chemical incorporation of drugs into poly(anhydride-ester) (PAE) backbones could solve most of the drawbacks associated with the controlled-release formulations mentioned above. In the last decade multiple non-steroidal anti-inflammatory drugs (e.g., salicylic acid and other salicylates) and antiseptics/antioxidants (e.g., catechol) have been chemically incorporated into PAE backbones.[23–31] These new classes of polymers are capable of achieving high drug loading (50–80 %) in a reproducible manner. The drug is chemically incorporated in each repeat unit through a “linker” molecule. These PAEs release the drug in a near zero-order fashion without a burst.[32–34] Drug release can be controlled by altering the chemical composition of the polymer (i.e., “linker” molecule or making copolymers).[26, 34–36] These PAEs are also advantageous because they can be formulated into different geometries depending on the intended administration route. For example, they can be formulated into microspheres for injectable administration.[37, 38]
Based upon our previous experience of incorporating drugs into PAE backbones, a morphine-based PAE was designed to control morphine release to achieve prolonged analgesia. This work presents the synthesis and characterization of this morphine-based PAE (termed “PolyMorphine”). The polymer was synthesized by melt-condensation polymerization and the chemical structure characterization was performed using proton and carbon nuclear magnetic resonance (1H- and 13C-NMR) spectroscopies, and infrared (IR) spectroscopy. The weight-average molecular weight (Mw) was determined by gel permeation chromatography (GPC), and the thermal properties were assessed using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Furthermore, in vitro studies were performed to study polymer degradation and drug release in buffered media mimicking physiological conditions, and cytocompatibility towards fibroblasts. In vivo studies of analgesia in mice were performed using tail-flick latency (TFL) tests.
2. Materials and Methods
2.1. Chemical and reagents
Morphine was kindly provided by Noramco Inc. (Athens, GA). Unless otherwise specified, all other chemicals and reagents were purchased from Sigma-Aldrich (Milwaukee, WI).
2.2. 1H-NMR and 13C-NMR and IR spectroscopies
1H- and 13C-NMR spectra were obtained using a Varian 500 MHz spectrometer. Samples were dissolved (~ 5 mg/mL for 1H-NMR and ~ 20 mg/mL for 13C-NMR) in deuterated dimethyl sulfoxide (DMSO-d6), which was used as an internal reference. Each spectrum was an average of 16 and 250 scans, respectively.
Fourier transform infrared (FT-IR) spectra were obtained using a Thermo Nicolet/Avatar 360 FT-IR spectrometer. Samples (1 wt%) were ground with KBr and compressed into a disk (13 mm diameter × 0.5 mm thick) using a hydraulic press (Carver model M) applying pressure (10,000 psi) for 1 min or solvent-cast onto NaCl plates using dichloromethane (DCM). Each spectrum was an average of 32 scans.
2.3. Molecular weight
Mass spectrometry [39] was used to determine the molecular weights (MW) of polymer intermediates. A Finnigan LCQ-DUO equipped with Xcalibur software and an adjustable Atmospheric Pressure ionization Electrospray Ion Source (API-ESI) was used. Samples were dissolved in methanol and diluted to 10 μg/mL before injection using a glass syringe. Pressure during the experiments was 0.8×10−5 Torr and the API temperature was 150 °C.
GPC was used to determine the Mw of the polymer. A Perkin-Elmer LC system consisting of a Series 200 refractive index detector, a Series 200 LC pump, and an ISS 200 advanced sample processor was used. A Dell OptiPlex GX110 computer running Perkin-Elmer TurboChrom 4 software was utilized for data collection and control. The connection between the LC system and the computer was made using a Perkin-Elmer Nelson 900 Series Interface and 600 Series Link. Samples were dissolved in DCM (10 mg/mL) and filtered through 0.45 μm polytetrafluoroethylene syringe filters (Fisher) prior to elution through a Jordi divinylbenzene mixed-bed GPC column (7.8 × 300 mm) (Alltech Associates, Deerfield, IL) at a rate of 1 mL/min for a total run time of 30 min. Weight-average molecular weights and polydispersity indexes (PDIs) were calculated relative to narrow Mw polystyrene standards (Polysciences, Dorval, Canada).
2.4. Thermal analysis
Thermal analysis was performed using DSC to obtain the glass transition (Tg) and melting (Tm) temperatures. DSC was performed using a Thermal Advantage (TA) DSC Q200 running on an IBM ThinkCentre computer equipped with TA Instrument Explorer software for data collection and control. Samples (4–8 mg) were heated under nitrogen from −10 °C to 200 °C at a heating rate of 10 °C/min. A minimum of two heating/cooling cycles were used for each sample set. TA Universal Analysis 2000, version 4.5A was used to analyze the data.
TGA was used to obtain the decomposition temperatures (Td). TGA analysis was performed using a Perkin-Elmer TGA7 analyzer with TAC7/DX controller equipped with a Dell OptiPlex Gx 110 computer running Perkin-Elmer Pyris software. Samples (~10 mg) were heated under nitrogen at a rate of 10 °C/min from 25 to 400 °C. Td was defined as the onset of decomposition and is represented by the beginning of a sharp slope on the thermogram.
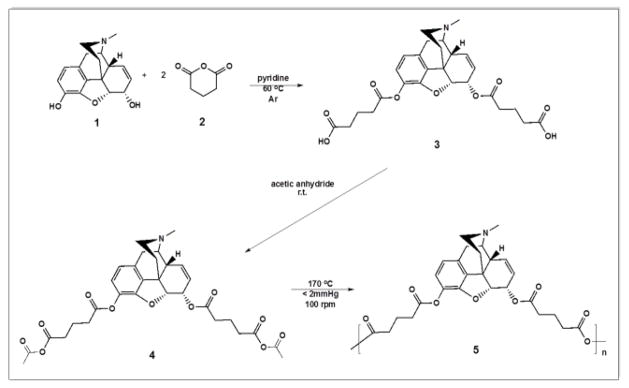
2.5. Diacid synthesis (3 in Scheme 1)
Scheme 1.
Synthesis of PolyMorphine 5 from the reaction of morphine 1 and glutaric anhydride via ring-opening, followed by acetylation of the diacid 3 and polymerization of the monomer 4 by melt-condensation.
Morphine (1 in Scheme 1, 1.00 g, 1 eq) was dissolved in anhydrous pyridine under argon and stirred for 5 min. Glutaric anhydride (2, 3.97 g, 10 eq) was slowly added manually. The reaction mixture was heated to 60 °C and stirred overnight. Pyridine was azeotropically removed using toluene. The brown paste obtained was washed 10 × 50 mL with DCM to remove the excess glutaric acid. The final product was dried under vacuum at room temperature. Yield: 0.95 g (95 %) beige foam. 1H-NMR (500 MHz, DMSO-d6, δ): 6.73 (d, 1H, ArH), 6.58 (d, 1H, ArH), 5.50 (dq, 2H, CH and CH), 5.15 (s, 1H, CH), 5.05 (d, 1H, CH), 3.37 (s, 1H, CH2), 2.98 (d, 1H, CH), 2.75 (s, 1H, CH), 2.40–2.15 (comp, 14H, CH2, CH2, CH2, CH2, CH2, and CH3), 2.08 (t, 1H, CH2), 1.86–1.68 (comp, 4H, CH2 and CH2), 1.65 (d, 1H, CH2).13C-NMR (500 MHz, DMSO-d6, δ): 174.1 (2C), 171.9 (1C), 170.5 (1C), 149.1 (1C), 131.5 (1C), 130.5 (1C), 130.3 (1C), 129.2 (1C), 127.8 (1C), 122.5 (1C), 119.7 (1C), 87.9 (1C), 67.4 (1C), 58.8 (1C), 45.8 (1C), 41.4 (1C), 40.8 (1C), 36.6 (1C), 32.9 (1C), 32.8 (1C), 32.6 (3C), 32.3 (1C), 32.9 (1C), 20.0 (1C). IR (KBr pellet): 3550 cm−1 (OH, acid), 1732 cm−1 (C=O, ester), 1712 cm−1 (C=O, acid). MS: 514 [M + 1]. Td = 227 °C.
2.6. Monomer synthesis (4 in Scheme 1)
Morphine-based diacid (3, 0.18 g) was acetylated by reacting with an excess of acetic anhydride (36 mL, Fisher, Fair Lawn, NJ). The reaction mixture was stirred overnight at room temperature. The excess acetic anhydride was removed under reduced pressure. Yield: 0.16 g (89 %), orange paste. 1H-NMR (500 MHz, DMSO-d6, δ): 6.74 (d, 1H, ArH), 6.59 (d, 1H, ArH), 5.50 (dq, 2H, CH and CH), 5.18 (s, 1H, CH), 5.05 (1H, CH), 5.05 (d, 1H, CH), 3.30 (s, 1H, CH2), 2.97 (d, 1H, CH), 2.78–2.12 (comp, 20H, CH, 5CH2 and 3CH3), 2.05 (t, 1H, CH2), 1.96–1.77 (comp, 4H, CH2 and CH2), 1.62 (d, 1H, CH2). 13C-NMR (500 MHz, DMSO-d6, δ): 172.2 (2C), 170.8 (2C), 169.2 (1C), 168.8 (1C), 145.0 (1C), 132.5 (1C), 132.3 (1C), 131.2 (1C), 131.1 (1C), 128.4 (1C), 122.3 (1C), 119.8 (1C), 89.7 (1C), 69.2 (1C), 58.4 (1C), 46.5 (1C), 43.4 (1C), 43.3 (1C), 35.4 (1C), 34.3 (1C), 34.2 (1C), 32.9 (3C), 32.5 (1C), 32.0 (1C), 30.0 (2C), 20.0 (1C). IR (solvent-casted DCM): 1809 cm−1 and 1761 cm−1 (C=O, anhydride), 1732 cm−1 (C=O, ester). MS: 598 [M + 1]. Tm = 164 °C. Td = 297 °C.
2.7. Polymer synthesis (5 in Scheme 1)
Morphine-based monomer (4, 1.00 g) was polymerized by melt-condensation polymerization at 170 °C, under constant vacuum (< 2 mmHg), and constant stirring (100 rpm) using an overhead mechanical stirrer (T-line laboratory stirrer, Talboys Engineering Corp., Montrose, PA). Polymerization continued until the mixture solidified (~ 30 min). The product was cooled to room temperature and dissolved in DCM (2 mL). The polymer was precipitated dropwise over excess diethyl ether (50 mL) and isolated by vacuum filtration. The product was dried under vacuum at room temperature overnight. Yield: 0.70 g (70 %), tan solid. 1H-NMR (500 MHz, DMSO-d6, δ): 6.71 (br, 1H, ArH), 6.55 (br, 1H, ArH), 5.50 (br, 2H, CH and CH), 5.15 (br, 1H, CH), 5.05 (br, 1H, CH), 3.29 (br, 1H, CH2), 2.93 (br, 1H, CH), 2.76–2.17 (br, 15H, CH2, CH2, CH2, CH2, CH2, CH2, and CH3), 2.00 (br, 1H, CH2), 1.93–1.67 (br, 4H, CH2 and CH2), 1.68 (br, 1H, CH2). 13C-NMR (500 MHz, DMSO-d6, δ): 175.1 (1C), 172.8 (1C), 172.6 (1C), 171.2 (1C), 149.9 (1C), 133.5 (1C), 132.4 (1C), 131.9 (1C), 130.8 (1C), 129.0 (1C), 122.5 (1C), 120.0 (1C), 89.3 (1C), 68.8 (1C), 58.8 (1C), 46.8 (1C), 43.5 (1C), 43.1 (1C), 35.4 (1C), 33.8 (1C), 33.5 (1C), 33.1 (3C), 32.9 (1C), 20.9 (1C). IR (solvent-casted DCM): 1818 cm−1 and 1761 cm−1 (C=O, anhydride), 1734 cm−1 (C=O, ester) Mw = 26,100 Da, PDI = 1.14. Tg = 120 °C. Td = 185 °C.
2.8. In vitro degradation studies
Diacid 3 (5.0 mg, triplicate) was placed into scintillation vials and 20.00 mL phosphate buffered saline (PBS) pH 7.4 added. Samples were incubated at 37 °C under constant shaking (60 rpm) in an Excella E25 Incubator Shaker (New Brunswick Scientific). PBS (1.00 mL) was removed at predetermined time points (2 h, 5 h, 10 h, and daily starting on day 1 for 30 days) and replaced with fresh PBS (1.00 mL). The pH was checked using an Accumet® Research AR15 pH meter (Fisher Scientific) and adjusted to 7.4 using 0.50 M NaOH when needed. Samples were immediately analyzed by HPLC.
For the polymer degradation studies, polymer 5 (5.0 mg, triplicate) was placed into scintillation vials and 20.00 mL phosphate buffered saline (PBS) pH 7.4 added. Samples were incubated at 37 °C under constant shaking (60 rpm) in an Excella E25 Incubator Shaker (New Brunswick Scientific). PBS (20.00 mL) was removed daily and replaced with fresh PBS. The pH was checked using an Accumet® Research AR15 pH meter (Fisher Scientific). Samples were immediately analyzed by HPLC.
2.9. High-performance liquid chromatography (HPLC)
Quantitative analysis of the in vitro degradation products was performed via HPLC using an XTerra® RP18 5 μm 4.6 × 150 mm column (Waters, Milford, MA) on a Waters 2695 Separations Module equipped with a Waters 2487 Dual λ Absorbance Detector. The system was connected to a Dell computer running Empower software. Samples were filtered using 0.22 μm poly(vinylidine fluoride) syringe filters (Fisher). The HPLC method was adapted from previously published methods.[40, 41] The mobile phase used was composed of 50 mM KH2PO4, 2.5 mM sodium dodecyl sulfate, 25 % acetonitrile, and 75% water at pH 3. Samples (20 μL) were run at 35 °C at a flow rate of 1 mL/min. Absorbance was monitored at λ = 210 nm. The instrument was calibrated using standard morphine 1 and diacid 3 solutions of known concentrations.
2.10. Cell cytocompatibility studies
Cytocompatibility was evaluated by culturing 3T3 fibroblasts cells (NIH 3T3 fibroblast cell line) in diacid- and/or polymer-containing medium at concentrations of 0.10 and 0.01 mg/mL. Cell culture medium consisted of Dulbecco’s modified Eagle’s medium (DMEM), 10 vol% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 1% l-glutamate, and 1% penicillin/streptomycin. Fibroblasts were seeded at a density of 2,000 cells/well in 96 well plates containing 150 μL of culture medium. The positive control consisted of fibroblasts with cell culture media only and the negative control consisted of fibroblasts with cell culture media and 5% 200-proof ethanol (PHARMCO-AAPER). Cells were incubated at 37 °C and 5% CO2 for 24, 48 and 72 h. Cell viability was determined using Calcein AM and ethidium homodimer-1 staining (Molecular Probes) according to the manufacturer’s protocol and the results normalized to the positive control. For each of the three time points (24, 48 and 72 h), a student’s t-test was performed to assess for statistical significance between the positive control and experimental conditions. Experiments were performed in quadruplicate.
2.11. In vivo animal studies
Adult male C57Bl/6J mice were obtained from Charles River (Kingston, NY). Animals were approximately 10 weeks old and weighed between 19.5 – 27.7 g at the beginning of the study. Animals were housed in climate-controlled rooms with a 12:12 hour light/dark cycle, with food and water available ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Rutgers University, and consistent with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 2011). Animals were pre-handled twice a day for 3 days prior to the experiment.
Polymer 5 (200.0 mg powder) was suspended in 10 mL of 5 % Cremophor EL in saline by vortex and stirred for 15 min. Diacid 3 (50.0 mg foam) and morphine HCl (10 mg) were each dissolved in 10 mL of 5 % Cremophor EL in saline. A 5 % Cremophor EL saline solution was used as the vehicle control. All administrations were by intraperitoneal (i.p.) injection. Drug dosing was as follows: free morphine (morphine HCl) at 10 mg/kg, 3 at 50 mg/kg, and 5 at 200 mg/kg.
Nociception in mice was measured with the TFL test. Animals were wrapped loosely in soft cloth, where each cage of animals had its own cloth to minimize cross-cage olfactory sensory stimulation. TFL was tested by immersing the distal third of the animal’s tail in a water bath at 49 °C, and the TFL time was recorded with a 30 s cutoff time to avoid tissue damage. Animals were only tested one time at each time point.
There were 30 animals in each group at the beginning of the study. TFL was measured at the following time points after the drug administration: 30 min, 1 h, 2 h, 4 h, 8 h, 1 d, 2 d, 3 d, 7 d, 9 d, and 14 d. On day 3, 15 animals from each group (including the vehicle control group) were tested for morphine sensitivity using the TFL test after by being subjected to an acute morphine dose (10 mg/kg of free morphine in 5 % Cremphor EL in saline). The remaining 15 animals continued to be tested as scheduled. On day 14, after being tested for TFL, all animals received an acute dose of morphine (10 mg/kg of free morphine) and tested for morphine sensitivity using the TFL test.
3. Results and Discussion
3.1. Synthesis and physicochemical characterization of PolyMorphine
In an effort to overcome the limitations of commercially available morphine delivery systems and based upon our experience with the chemical incorporation of drugs into biodegradable polymer backbones, a morphine-based PAE, described herein as PolyMorphine (5 in Scheme 1), was developed and evaluated. The synthesis of this polymeric prodrug consists of three steps as outlined in Scheme 1: esterification of morphine to yield the diacid (3), which is then activated via acetylation to form the monomer (4) that undergoes melt-condensation polymerization to yield the polymer (5). All compounds synthesized were characterized to assess their physical and chemical properties. Their chemical structures were assessed using 1H- and 13C-NMR, and FT-IR spectroscopy. MS and GPC were used to determine the MW and Mw, respectively. The thermal properties were evaluated using DSC and TGA.
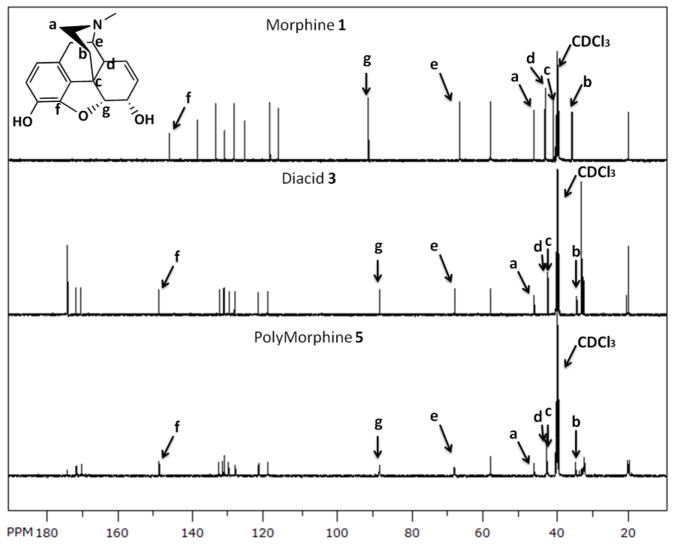
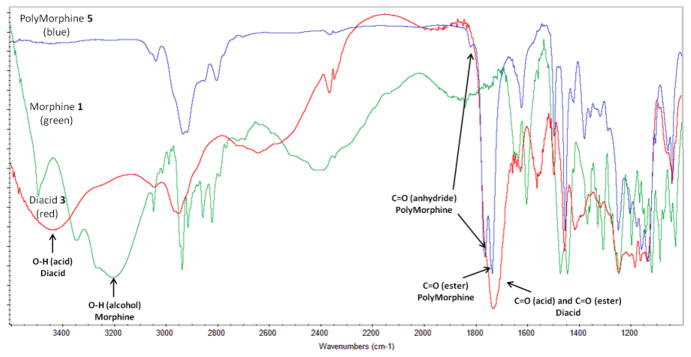
To synthesize 3, various reaction conditions were explored by changing the solvent and the base catalyst. Among the conditions tested, the reaction carried out neat in pyridine yielded the best results (i.e., full conversion into product and easy product isolation). Because the allylic hydroxyl group of morphine is less reactive than the phenolic alcohol, the complete conversion of both alcohols takes 3 days at room temperature. When heated to 60 °C, esterification of the phenolic and allylic alcohols is completed within 24 hours. The isolation of the product was performed by azeotropic removal of pyridine with toluene to reproducibly afford 3 in high yields (95 %). Figure 1 shows the 13C-NMR of 1, 3, and 5; the key peaks for the nitrogen-containing ring and the cyclic ether are indicated. As shown in Figure 1, the structure of the drug was preserved after synthesizing 3. The IR spectrum of 3 (Figure 2, red) shows the attachment of glutaric linkers by the formation of the ester bonds by the presence of the ester carbonyl (C=O) at 1732 cm−1 and the presence of terminal carboxylic acids C=O at 1712 cm−1 and O-H at 3350 cm−1. Compared to the IR spectrum of morphine (Figure 2, green), the alcohols O-H at 3200 cm−1 disappear and the C=O peaks appear. The MW of 3 was determined as 514 by MS, which corresponds to the MW of 3 (513.54) plus a proton. The thermal analysis of 3 showed that it decomposes at 227 °C and did not display a Tm.
Figure 1.
13C-NMR spectra of morphine 1, diacid 3, and PolyMorphine 5, showing the preservation of the chemical integrity of the drug; key peaks for the nitrogen-containing ring and the cyclic ether are indicated.
Figure 2.
Infrared spectra of (blue) PolyMorphine 5, (red) diacid 3, and (green) morphine 1, key stretch bands for OH acid, C=O acid, C=O ester, and C=O anhydride are indicated.
Two different polymerization methods were investigated to prepare PolyMorphine. Due to the concern that morphine intermediates might be thermally unstable, solution polymerization was first evaluated. This method used triphosgene (which forms phosgene in situ) as the coupling agent in the presence of triethylamine.[42] However, this polymerization method not only resulted in low Mw polymer and low yields, but the pure polymer could not be isolated. As a result, melt-condensation polymerization was attempted.[42] Monomer 4 was prepared by the acetylation of 3 in excess acetic anhydride at room temperature. Characterization of 4 was performed with the same methods used to characterize 3; the NMR and IR spectra confirmed the formation of 4. Monomer 4 decomposes at 297 °C and melts at 164 °C. This high Td of 4 and its moderate Tm made melt-condensation polymerization possible because it was thermally stable.
Melt-condensation polymerization of activated 4 at 170 °C in vacuo yielded 5 with reasonably high Mw (26,000 Da), low PDI (1.14) and high yields (70 %). Figure 1 also shows the 13C-NMR spectrum of 5, as seen on the figure the structure of the drug was preserved. The IR spectrum of 5 (Figure 2, blue) shows the formation of the anhydride bonds by the presence of the anhydride C=O at 1818 and 1761 cm−1, the preservation of the ester bonds by the presence of the ester C=O at 1734 cm−1, and the disappearance of terminal carboxylic acid C=O at 1712 cm−1. PolyMorphine 5 decomposes at 185 °C, does not have a Tm, and its Tg is 120 °C. Having such a high Tg is a positive attribute for in vivo applications (body temperature is 37 °C) because the polymer will not deform once implanted in the body.
3.2. In vitro degradation and drug release
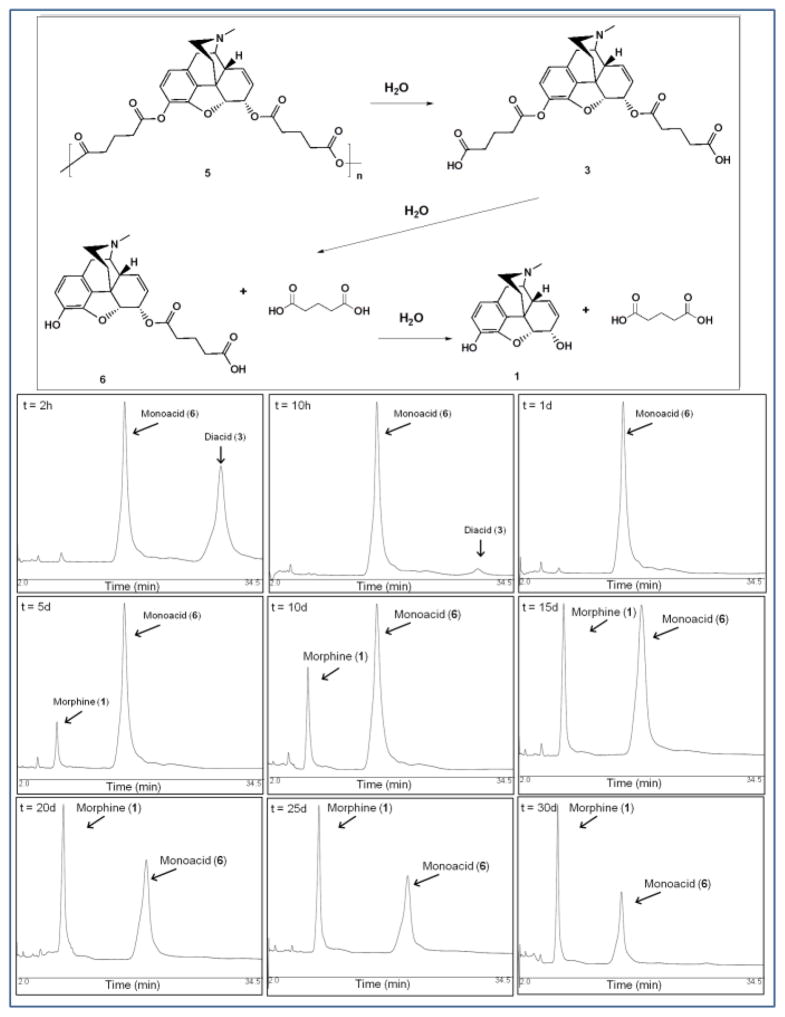
Given that 5 was designed to degrade and release free morphine, in vitro hydrolysis studies were performed to characterize polymer degradation (Figure 3). Since the hydrolytic cleavage of the anhydride bonds is faster than the ester bonds,[43, 44] the degradation of 3 was expected to be the rate-determining step in the degradation of 5. In addition, the two ester bonds in compound 3 are not equivalent and would likely degrade at different rates. Diacid 3 is an important intermediate; if it does not degrade to release free morphine, then polymer 5 will not degrade into free morphine.
Figure 3.
[14] Hydrolytic degradation scheme of PolyMorphine (5). (Bottom) Chromatograms showing the in vitro degradation of diacid (3) into monoacid (6) and free morphine (1) at different time points (2 h, 10 h, 1 d, 5 d, 10 d, 15 d, 20 d, 25 d, and 30 d).
Mimicking physiological conditions (37 °C and pH 7.4 buffer), the hydrolytic degradation of 3 was analyzed by HPLC where three distinctive peaks were detected throughout the experiment: 3 (Rt = 28.5 min), 6 (Rt = 16.2 min), and 1 (Rt = 6.5 min). Figure 3 (bottom) shows representative chromatograms for the degradation of 3 into the intermediate 6 and 1. Diacid 3 completely hydrolyzes into a monoacid (Figure 3 top, 6) during the first day. The monoacid then hydrolyzed into free morphine (that started to be detected on day 2) and was still present after 30 days (Figure 3 bottom). The formation of 6 during degradation was confirmed by the analysis of the chemically synthesized monoacid 7 (supplementary data, Figure 1S). The retention time of 7 was 18.1 min, which is different from that of 6. When both monoacids were analyzed simultaneously, a peak with two maximums was observed; the low resolution suggests the presence of two similar compounds. This degradation pathway is supported by previous studies on the hydrolysis of heroin into 6-monoacetylmorphine and ultimately into morphine.[45]
Following analysis of 3, the hydrolytic degradation of 5 was studied under similar conditions. The HPLC results indicated that the polymer degrades via hydrolytic cleavage of the anhydride bonds to generate 3, which is then hydrolyzed into 6, which further hydrolyzes into 1 (Figure 3 top).
3.3. In vitro cytocompatibility
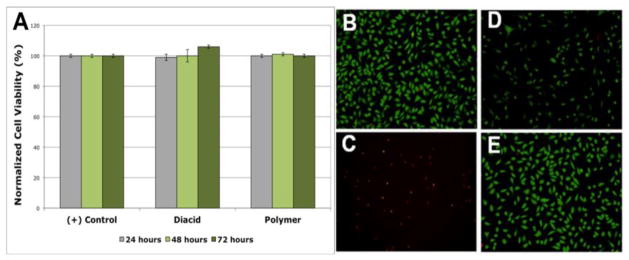
Investigating the potential toxicity of these novel materials is critical to understanding the potential in vivo use of this prodrug. The cytotoxicity of 3 and 5 towards fibroblasts was studied in vitro. Fibroblasts were used for this study because they are the most frequently used cells for initial cytotoxicity testing of biomaterials.[46] Cytocompatibility was evaluated by culturing 3T3 fibroblasts cells in medium containing 3 and 5 (separately) at concentrations of 0.10 and 0.01 mg/mL. These concentrations were chosen because they are well above the concentrations seen in vitro (10–100 times higher) and can be used to determine a possible dose dependent toxicity. Studies were performed evaluating cell viability at 24, 48, and 72 h, to evaluate early and late degradation stages. To quantify cell viability, representative fluorescence microscopy images of each condition were taken to determine the total number of cells (live and dead). Statistical analysis showed no significant differences with a 95 % confidence level between the samples containing 3 and 5 and the positive control for both concentrations used at all time points. Comparison between the diacid- and polymer-containing samples and the media control indicate normal to higher cell viability, suggesting that both 3 and 5 are non-cytotoxic (Figure 4A). Figure 4(B–D) shows representative fluorescence microscopy images of the positive control (fibroblasts with cell culture media), the negative control (fibroblasts with cell culture media and 5 % ethanol), cell culture media containing 3 (0.10 mg/mL at 48 h), and cell culture media containing 5 (0.10 mg/mL at 48 h). Green fluorescence indicates viable cells whereas red indicates dead cells. These results show no significant cytotoxicity caused by 5 or 3.
Figure 4.
In vitro cell cytocompatibility of diacid (3) and PolyMorphine (5). (A) Cell viability of the positive control (fibroblasts with cell culture media only), 3 (at 0.10 mg/mL), and 5 (at 0.10 mg/mL), no statistical differences at 95 % confidence level between the samples containing 3 and 5 and the positive control; Fluorescent microscopy images (green = viable cell and red = dead cells) of: (B) positive control, (C) negative control (fibroblasts with cell culture media and 5 % ethanol), (D) diacid 3, and (E) 5.
3.4. In vivo evaluation of analgesic effect
As indicated above, a key impetus of this work was to develop a prodrug form of morphine (PolyMorphine), which, when administered in vivo, would hydrolytically degrade in a controlled fashion to provide extended analgesia. To determine whether 5 would meet this objective, mice were administered systemically with a drug or control solution by i.p. injection, and their nociception was measured using the TFL test. TFL test was performed by immersing the distal third of the animal’s tail in a water bath at 49 °C and measuring pain threshold by the time it takes for the animal to flick its tail. Four treatment groups were used: vehicle control, free morphine (at 10 mg/kg), 3 (at 50 mg/kg), and 5 (at 200 mg/kg). Doses were chosen after a pilot dose-response experiment. Doses do not contain the same amount of morphine, however, higher concentration of morphine after a single administration does not result in an extended analgesic effect.[47] At various time points post administration (starting after 30 min), TFL was measured.
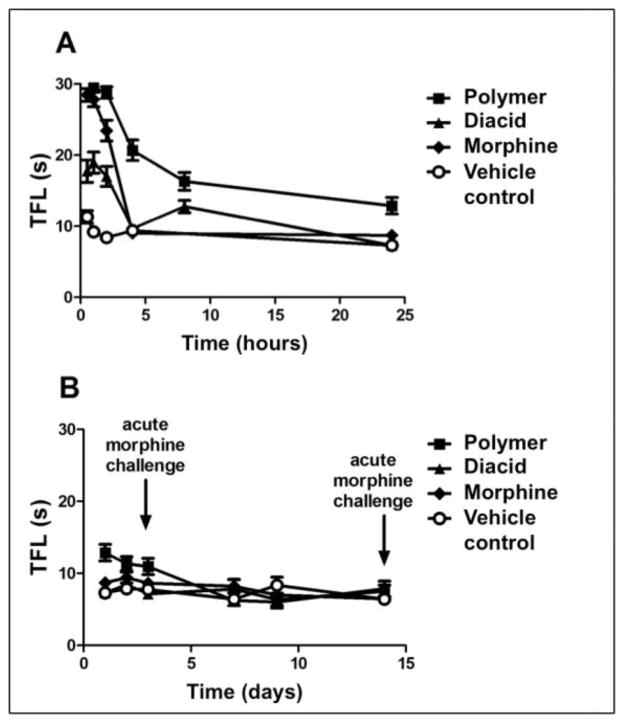
As shown in Figure 5, free morphine provided strong analgesia, peaking at 30 min post-administration (Figure 5A, filled diamonds). The analgesic effect of free morphine diminished with time; by the 4 h time point, the analgesic effect was completely gone. This time course of analgesia has been well-established for free morphine, as the drug is metabolized in vivo and plasma drug level drops off.[8] Diacid (3) showed a similar time course of analgesic effect as free morphine (Figure 5A, filled triangles).
Figure 5.
PolyMorphine provided extended analgesia in mice. (A) TFL test results at 0.5–24 h post-administration. (B) TFL results from day 1 through day 14 (vertical arrows indicate the days that animals received acute morphine challenge to evaluate morphine tolerance development). PolyMorphine provides extended analgesia compared with free morphine. Data are shown as mean ± standard error of mean. N = 30 for each time point prior to and including day 3. N = 15 after day 3.
Systemic administration of PolyMorphine (5) also resulted in strong analgesia, reaching a peak effect at the 1 h time point (Figure 5A, filled squares). Different from free morphine, however, is the noticeably extended time course of the analgesic effect from PolyMorphine. Analgesia was sustained throughout the 24 h time frame post drug administration with gradual decline (Figure 5A), with the analgesic effect still detectable 3 days post-administration (Figure 5B). These results clearly indicate that PolyMorphine, when administered in vivo, provides extended pain relief. The fact that analgesia was detectable 3 days post-administration was note-worthy; this study is the first example of a single dose, systemically administered morphine formulation that displayed analgesia for over 24 h.
Compared to the in vitro drug release studies, hydrolysis of the polymer seems to be faster in vivo. As morphine, monoacid 6, and diacid 3 are detected during in vitro degradation studies, it is possible that the analgesic effect comes from all compounds. It was already shown that administration of 3 results in analgesia. Therefore, further studies are needed to determine the concentration in blood of each degradation product at each time point.
In opioid biology, a well-known effect of the extended use of morphine (and related opioid alkaloids with strong analgesic properties), both in rodent and human, is tolerance development with repeated exposure.[39, 48, 49] As a preliminary evaluation of animals’ sensitivity to acute morphine, two time points were chosen at which the animals’ responsiveness to an acute morphine challenge was tested. If animals became morphine-tolerant, they would be less responsive to a morphine challenge (administration of 10mg/kg of morphine). The first time point was 3 days post-drug administration, as this was the time when PolyMorphine’s analgesic effect has decreased substantially toward the baseline level. Half of the mice from each drug group were subjected to acute morphine challenge on day 3. The second time point was on day 14, when the remaining half of the mice from each experimental group were subjected to acute morphine challenges. Mice in every group showed full responsiveness to acute morphine challenge, at both day 3 and day 14, reaching the 30 s cutoff time in TFL test. It should be noted that, although this preliminary assessment suggested an absence of overt morphine tolerance, more extensive work is needed to fully evaluate the issue of morphine tolerance.
4. Conclusion
This study reports the preparation and evaluation of PolyMorphine, a polymer version of morphine that provides extended analgesia while potentially reducing tolerance development. PolyMorphine was synthesized via melt-condensation polymerization and its physicochemical properties were fully characterized to confirm the preservation of morphine’s structural integrity. In vitro studies were performed to determine the degradation pathway of the polymer and a key intermediate, showing that PolyMorphine hydrolyzes into free morphine. In vitro cytocompatibility studies showed that PolyMorphine is non-cytotoxic towards fibroblasts. When administered in vivo, PolyMorphine provided sustained pain relief for up to 3 days, more than 20 times the analgesic time window of free morphine. These results demonstrated, for the first time, a systemically administered prodrug that yields such a long-lasting analgesic effect. Furthermore, based on a preliminary test of sensitivity to an acute morphine challenge, no overt signs of morphine tolerance development were observed in PolyMorphine-administered animals. In consideration of the abuse liability of many controlled release formulations of opioid analgesics, PolyMorphine may offer a desirable option as a long-acting, low abuse liability alternative to conventional opioid analgesics. Clearly, these potential promises warrant further investigation.
Supplementary Material
Acknowledgments
The authors thank the National Institutes of Health (NIH 5 R01DE0132070-09 and NIH 1 R01DE019926-01), the Graduate Assistance in Areas of National Need (GAANN) Fellowship, and the Johnson & Johnson and Rutgers: Proof-of-Concept Fund for financial support. Bryan Langowski, David Orban, and Michael Drahl (Rutgers, Department of Chemistry & Chemical Biology) are thanked for intellectual discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakurada T, Komatsu T, Sakurada S. Mechanisms of Nociception Evoked by Intrathecal High-dose Morphine. NeuroToxicology. 2005;26:801–809. doi: 10.1016/j.neuro.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Portenoy RK, Sciberras Aa, Eliot L, Loewen G, Butler J, Devane J. Steady-State Pharmacokinetic Comparison of a New, Extended-Release, Once-Daily Morphine Formulation, Avinza®, and a Twice-Daily Controlled-Release Morphine Formulation in Patients with Chronic Moderate-to-Severe Pain. Journal of Pain and Symptom Management. 2002;23:292–300. doi: 10.1016/s0885-3924(02)00382-2. [DOI] [PubMed] [Google Scholar]
- 3.Vermeire A, Remon JP. Stability and compatibility of morphine. International Journal of Pharmaceutics. 1999;187:17–51. doi: 10.1016/s0378-5173(99)00181-7. [DOI] [PubMed] [Google Scholar]
- 4.Davis MP. Management of Cancer Pain: Focus on New Opioid Analgesic Formulations. American Journal of Cancer. 2006;5:171–182. [Google Scholar]
- 5.King CR, Khabazian A. Cancer treatments. AVINZA (morphine sulfate extended-release capsules) Clinical Journal of Oncology Nursing. 2003;7:458. doi: 10.1188/03.CJON.458-460. [DOI] [PubMed] [Google Scholar]
- 6.Ross EL, Hahn K. KADIAN® (morphine sulfate extended-release) Capsules for treatment of chronic, moderate-to-severe, nonmalignant pain. International Journal of Clinical Practice. 2008;62:471–479. doi: 10.1111/j.1742-1241.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- 7.Hagen NA, Thirlwell M, Eisenhoffer J, Quigley P, Harsanyi Z, Darke A. Efficacy, safety, and steady-state pharmacokinetics of once-a-day controlled-release morphine (MS Contin XL®) in cancer pain. Journal of Pain and Symptom Management. 2005;29:80–90. doi: 10.1016/j.jpainsymman.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Olsson B, Wagner ZG, Mansson P, Ragnarsson G. A gamma scintigraphic study of the absorption of morphine from controlled-release tablets. International Journal of Pharmaceutics. 1995;119:223–229. [Google Scholar]
- 9.Kim T, Kim J, Kim S. Extended-release formulation of morphine for subcutaneous administration. Cancer Chemotherapy and Pharmacology. 1993;33:187–190. doi: 10.1007/BF00686214. [DOI] [PubMed] [Google Scholar]
- 10.Chao J. Retrospective Analysis of Kadian® (Morphine Sulfate Sustained-Release Capsules) in Patients with Chronic, Nonmalignant Pain. Pain Medicine. 2005;6:262–265. doi: 10.1111/j.1526-4637.2005.05033.x. [DOI] [PubMed] [Google Scholar]
- 11.Smith HS. Enteral Controlled-Release Opioid Delivery Systems. Pain Medicine. 2009;10:S30–S38. [Google Scholar]
- 12.Grant GJ, Vermeulen K, Zakowski MI, Stenner M, Turndorf H, Langerman L. Prolonged Analgesia and Decreased Toxicity with Liposomal Morphine in a Mouse Model. Anesthesia & Analgesia. 1994;79:706–709. doi: 10.1213/00000539-199410000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Wang JJ, Sung KC, Yeh CH, Fang JY. The delivery and antinociceptive effects of morphine and its ester prodrugs from lipid emulsions. International Journal of Pharmaceutics. 2008;353:95–104. doi: 10.1016/j.ijpharm.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Küchler S, Wolf NB, Heilmann S, Weindl G, Helfmann J, Yahya MM, Stein C, Schäfer-Korting M. 3D-Wound healing model: Influence of morphine and solid lipid nanoparticles. Journal of Biotechnology. 2010;148:24–30. doi: 10.1016/j.jbiotec.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Polard E, Le Corre P, Chevanne F, Le Verge R. In vitro and in vivo evaluation of polylactide and polylactide-co-glycolide microspheres of morphine for site-specific delivery. International Journal of Pharmaceutics. 1996;134:37–46. [Google Scholar]
- 16.Morales ME, López G, Gallardo V, Ruiz MA. Oral Suspensions of Morphine Hydrochloride for Controlled Release: Rheological Properties and Drug Release. Molecular Pharmaceutics. 2011;8:629–634. doi: 10.1021/mp200019q. [DOI] [PubMed] [Google Scholar]
- 17.Morales ME, Gallardo Lara V, Calpena AC, Doménech J, Ruiz MA. Comparative study of morphine diffusion from sustained release polymeric suspensions. Journal of Controlled Release. 2004;95:75–81. doi: 10.1016/j.jconrel.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Arias JL, Gómez-Gallo A, Delgado ÁV, Ruiz MA. Kollidon® SR colloidal particles as vehicles for oral morphine delivery in pain treatment. Colloids and Surfaces B: Biointerfaces. 2009;70:207–212. doi: 10.1016/j.colsurfb.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Arévalo M, Alvarez-Fuentes J, Iruin A, Holgado MA. In vitro evaluation of a morphine polymeric complex: flowability behavior and dissolution study. AAPS PharmSciTech. 2004;5:e39. doi: 10.1208/pt050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holgado MA, Iruin A, Alvarez-Fuentes J, Fernández-Arévalo M. Development and in vitro evaluation of a controlled release formulation to produce wide dose interval morphine tablets. European Journal of Pharmaceutics and Biopharmaceutics. 2008;70:544–549. doi: 10.1016/j.ejpb.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Fuentes J, Fernández-Arévalo M, Holgado MA, Caraballo I, Rabasco AM, Micó JA, Rojas O, Ortega-Alvaro A. Preclinical study of a controlled release oral morphine system in rats. International Journal of Pharmaceutics. 1996;139:237–241. [Google Scholar]
- 22.Mahkam M, Sharifi-Sanjani N. Preparation of new biodegradable polyurethanes as a therapeutic agent. Polymer Degradation and Stability. 2003;80:199–202. [Google Scholar]
- 23.Erdmann L, Uhrich KE. Synthesis and degradation characteristics of salicylic acid-derived poly(anhydride-esters) Biomaterials. 2000;21:1941–1946. doi: 10.1016/s0142-9612(00)00073-9. [DOI] [PubMed] [Google Scholar]
- 24.Schmeltzer RC, Anastasiou TJ, Uhrich KE. Optimized Synthesis of Salicylate-based Poly(anhydride-esters) Polymer Bulletin. 2003;49:441–448. doi: 10.1007/s00289-003-0123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmeltzer RC, Schmalenberg KE, Uhrich KE. Synthesis and Cytotoxicity of Salicylate-Based Poly(anhydride esters) Biomacromolecules. 2004;6:359–367. doi: 10.1021/bm049544+. [DOI] [PubMed] [Google Scholar]
- 26.Prudencio A, Schmeltzer RC, Uhrich KE. Effect of Linker Structure on Salicylic Acid-Derived Poly(anhydride-esters) Macromolecules. 2005;38:6895–6901. doi: 10.1021/ma048051u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmeltzer RC, Uhrich KE. Synthesis and characterization of antiseptic-based poly(anhydride-esters) Polymer Bulletin. 2006;57:281–291. doi: 10.1007/s00289-006-0561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prudencio A, Carbone AL, Griffin J, Uhrich KE. A Novel Approach for Incorporation of Mono-Functional Bioactive Phenols into Polyanhydrides. Macromolecular Rapid Communications. 2009;30:1101–1108. doi: 10.1002/marc.200900059. [DOI] [PubMed] [Google Scholar]
- 29.Anastasiou TJ, Uhrich KE. Aminosalicylate-based biodegradable polymers: Syntheses and in vitro characterization of poly(anhydride-ester)s and poly(anhydride-amide)s. Journal of Polymer Science Part A: Polymer Chemistry. 2003;41:3667–3679. [Google Scholar]
- 30.Carbone AL, Song M, Uhrich KE. Iodinated Salicylate-Based Poly(anhydride-esters) as Radiopaque Biomaterials. Biomacromolecules. 2008;9:1604–1612. doi: 10.1021/bm8000759. [DOI] [PubMed] [Google Scholar]
- 31.Kim Y, Uhrich KE. Synthesis and characterization of 5-aminosalicylic acid based poly(anhydride-esters) by solution polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 2010;48:6003–6008. doi: 10.1002/pola.24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitaker-Brothers K, Uhrich K. Investigation into the erosion mechanism of salicylate-based poly(anhydride-esters) Journal of Biomedical Materials Research Part A. 2006;76A:470–479. doi: 10.1002/jbm.a.30356. [DOI] [PubMed] [Google Scholar]
- 33.Johnson ML, Uhrich KE. Concurrent release of admixed antimicrobials and salicylic acid from salicylate-based poly(anhydride-esters) Journal of Biomedical Materials Research Part A. 2009;91A:671–678. doi: 10.1002/jbm.a.32288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitaker-Brothers K, Uhrich K. Poly(anhydride-ester) fibers: Role of copolymer composition on hydrolytic degradation and mechanical properties. Journal of Biomedical Materials Research Part A. 2004;70A:309–318. doi: 10.1002/jbm.a.30083. [DOI] [PubMed] [Google Scholar]
- 35.Schmeltzer RC, Uhrich KE. Synthesis and Characterization of Salicylic Acid-Based Poly(Anhydride-Ester) Copolymers. Journal of Bioactive and Compatible Polymers. 2006;21:123–133. doi: 10.1177/0883911506062976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carbone AL, Uhrich KE. Design and Synthesis of Fast-Degrading Poly(anhydride-esters) Macromolecular Rapid Communications. 2009;30:1021–1026. doi: 10.1002/marc.200900029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeagy BA, Prudencio A, Schmeltzer RC, Uhrich KE, Cook TJ. Characterization and in vitro degradation of salicylate-derived poly(anhydride-ester) microspheres. Journal of Microencapsulation. 2006;23:643–653. doi: 10.1080/02652040600776481. [DOI] [PubMed] [Google Scholar]
- 38.Rosario-Meléndez R, Ouimet MA, Uhrich KE. Formulation of salicylate-based poly(anhydride-ester) microspheres for short- and long-term salicylic acid delivery. Polymer Bulletin. 2012 doi: 10.1007/s00289-012-0839-2. Manuscript accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams JT, Christie MJ, Manzoni O. Cellular and Synaptic Adaptations Mediating Opioid Dependence. Physiological Reviews. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 40.Gerostamoulos J, Drummer OH. Solid phase extraction of morphine and its metabolites from postmortem blood. Forensic Science International. 1996;77:53–63. doi: 10.1016/0379-0738(95)01833-6. [DOI] [PubMed] [Google Scholar]
- 41.Meng QC, Soleded Cepeda M, Kramer T, Zou H, Matoka DJ, Farrar J. High-performance liquid chromatographic determination of morphine and its 3- and 6-glucuronide metabolites by two-step solid-phase extraction. Journal of Chromatography B: Biomedical Sciences and Applications. 2000;742:115–123. doi: 10.1016/s0378-4347(00)00146-8. [DOI] [PubMed] [Google Scholar]
- 42.Schmeltzer RC, Johnson M, Griffin J, Uhrich K. Comparison of salicylate-based poly(anhydride-esters) formed via melt-condensation versus solution polymerization, Journal of Biomaterials Science. Polymer Edition. 2008;19:1295–1306. doi: 10.1163/156856208786052362. [DOI] [PubMed] [Google Scholar]
- 43.Achim Gp. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- 44.Siepmann J, Gopferich A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Advanced Drug Delivery Reviews. 2001;48:229–247. doi: 10.1016/s0169-409x(01)00116-8. [DOI] [PubMed] [Google Scholar]
- 45.Kamendulis LM, Brzezinski MR, Pindel EV, Bosron WF, Dean RA. Metabolism of cocaine and heroin is catalyzed by the same human liver carboxylesterases. Journal of Pharmacology and Experimental Therapeutics. 1996;279:713–717. [PubMed] [Google Scholar]
- 46.Dumitriu S. Polymeric Biomaterials. 2. Mercel Dekker Inc; New York, NY: 2002. [Google Scholar]
- 47.Lugo RA, Kern SE. Clinical Pharmacokinetics of Morphine. Journal of Pain and Palliative Care Pharmacotherapy. 2002;16:5–18. doi: 10.1080/j354v16n04_02. [DOI] [PubMed] [Google Scholar]
- 48.Harrison LM, Kastin AJ, Zadina JE. Opiate tolerance and dependence: receptors, G-proteins, and antiopiates. Peptides. 1998;19:1603–1630. doi: 10.1016/s0196-9781(98)00126-0. [DOI] [PubMed] [Google Scholar]
- 49.Ueda M, Fau H, Ueda UM. Mechanisms underlying morphine analgesic tolerance and dependence. Front Biosci. 2009;14:5260–5272. doi: 10.2741/3596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.