Abstract
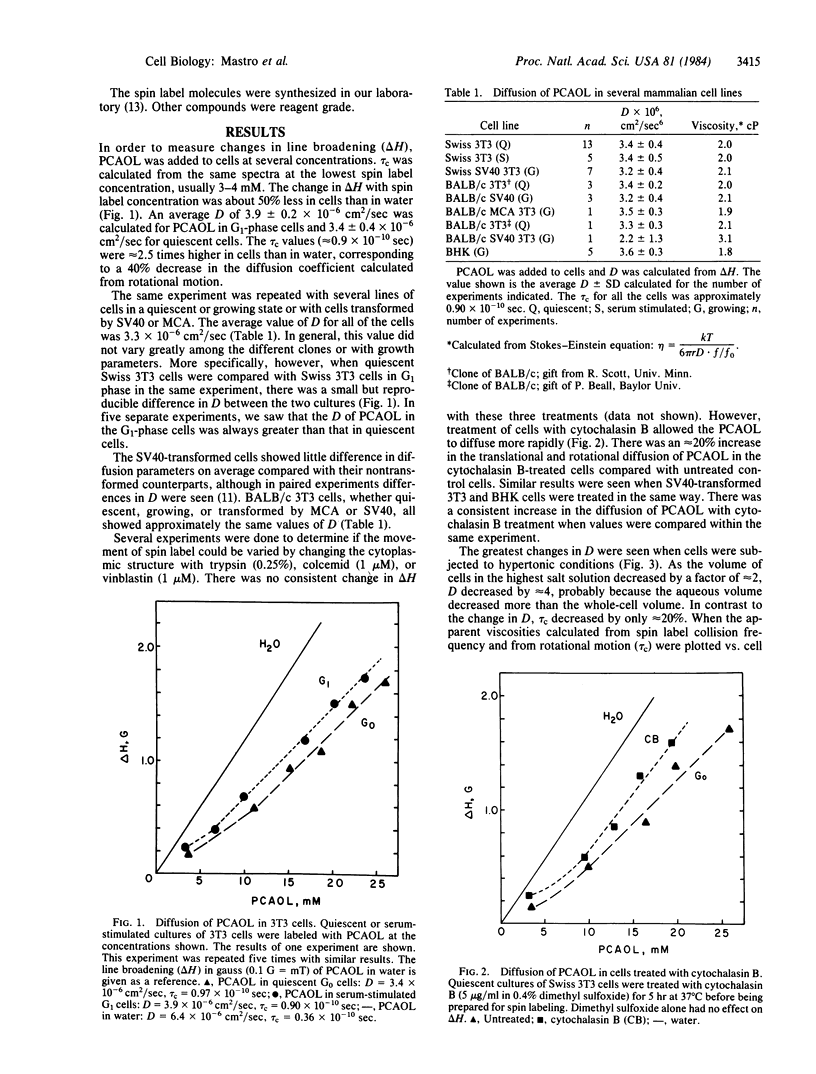
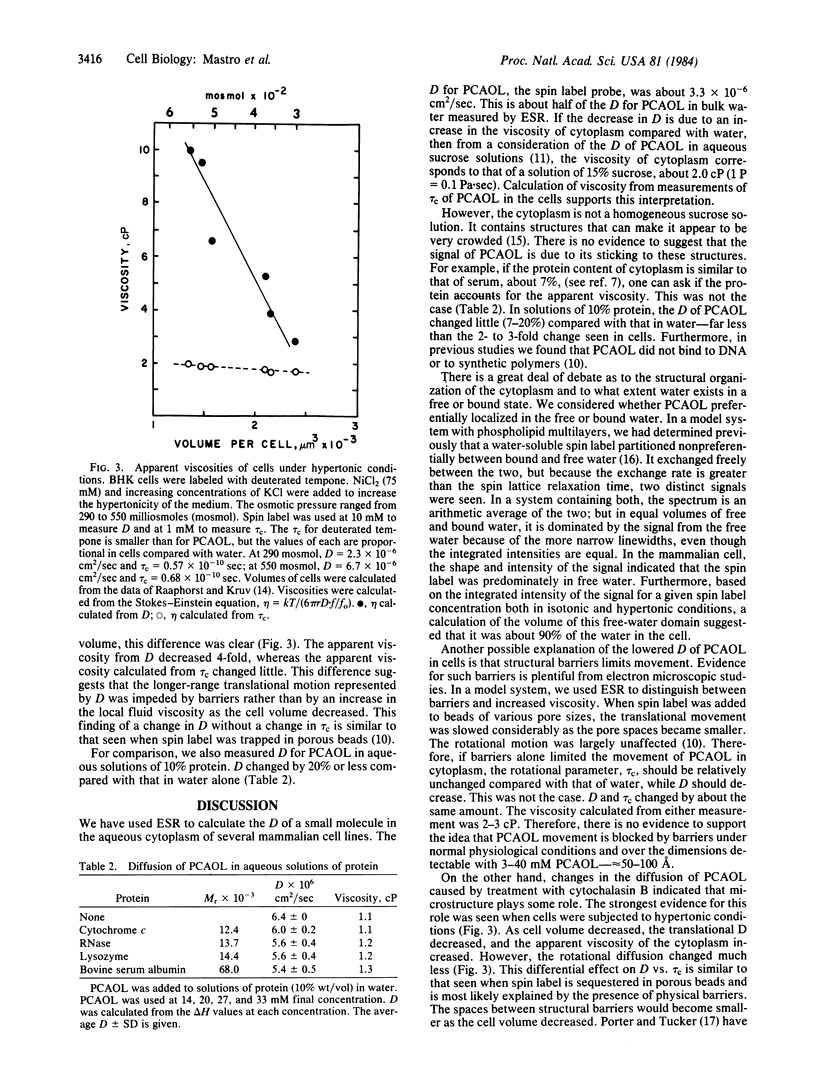
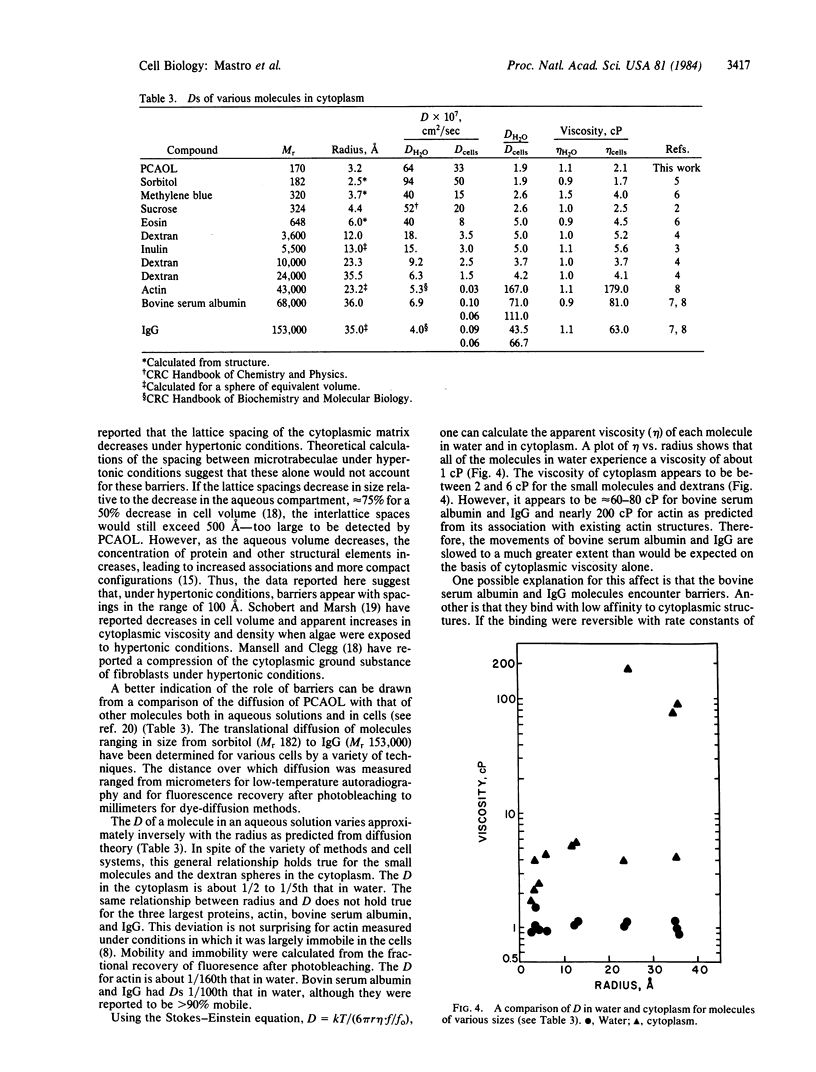
Electron spin resonance was used to measure the diffusion of a small (Mr 170) spin label in the aqueous cytoplasm of mammalian cells. Translational and rotational motion were determined from the same spectra. Based on measurements made in model systems, it was hypothesized that calculations of the apparent viscosity from either rotational or translational motion would distinguish between the effects of cytoplasmic viscosity or cytoplasmic structure on diffusion. The diffusion coefficient calculated from spin label collision frequency, averaged 3.3 X 10(-6) cm2/sec in several cell lines. It was greater in growing cells and in cells treated with cytochalasin B than in quiescent cells. The viscosity of the cytoplasm calculated from the translational diffusion coefficient or the rotational correlation time was 2.0-3.0 centipoise (1 P = 0.1 Pa X sec), about 2-3 times that of the spin label in water. Therefore, over the dimensions measured by the technique, 50-100 A, solvent viscosity appears to be the major determinant of particle movement in cells under physiological conditions. However, when cells were subjected to hypertonic conditions, the translational motion decreased by 67%, while the rotational motion changed less than 20%. These data suggested that the decrease in cell volume under hypertonic conditions was accompanied by an increase in cytoplasmic barriers and a decrease in the spacing between existing components. In addition, a comparison of reported values for diffusion of a variety of molecules in water and in cells indicates that cytoplasmic structure plays an important role in the diffusion of proteins such as bovine serum albumin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caillé J. P., Hinke J. A. The volume available to diffusion in the muscle fiber. Can J Physiol Pharmacol. 1974 Aug;52(4):814–828. doi: 10.1139/y74-107. [DOI] [PubMed] [Google Scholar]
- Fulton A. B. How crowded is the cytoplasm? Cell. 1982 Sep;30(2):345–347. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerstedt R. H., Keith A. D., Boltz R. C., Jr, Todd P. W. Use of amphiphilic spin labels and whole cell isoelectric focusing to assay charge characteristics of sperm surfaces. Arch Biochem Biophys. 1979 May;194(2):565–580. doi: 10.1016/0003-9861(79)90652-0. [DOI] [PubMed] [Google Scholar]
- Horowitz S. B., Fenichel I. R., Hoffman B., Kollmann G., Shapiro B. The intracellular transport and distribution of cysteamine phosphate derivatives. Biophys J. 1970 Oct;10(10):994–1010. doi: 10.1016/S0006-3495(70)86348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S. B., Moore L. C. The nuclear permeability, intracellular distribution, and diffusion of inulin in the amphibian oocyte. J Cell Biol. 1974 Feb;60(2):405–415. doi: 10.1083/jcb.60.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S. B. The permeability of the amphibian oocyte nucleus, in situ. J Cell Biol. 1972 Sep;54(3):609–625. doi: 10.1083/jcb.54.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith A. D., Snipes W., Chapman D. Spin-label studies on the aqueous regions of phospholipid multilayers. Biochemistry. 1977 Feb 22;16(4):634–641. doi: 10.1021/bi00623a013. [DOI] [PubMed] [Google Scholar]
- Keith A. D., Snipes W., Mehlhorn R. J., Gunter T. Factors restricting diffusion of water-soluble spin labels. Biophys J. 1977 Sep;19(3):205–218. doi: 10.1016/S0006-3495(77)85582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith A. D., Snipes W. Viscosity of cellular protoplasm. Science. 1974 Feb 15;183(4125):666–668. doi: 10.1126/science.183.4125.666. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Geiger B., Schlessinger J. Mobility of microinjected rhodamine actin within living chicken gizzard cells determined by fluorescence photobleaching recovery. Cell. 1982 Jul;29(3):835–845. doi: 10.1016/0092-8674(82)90445-7. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Cheng K. H., Campbell S. D., Kruuv J. Rotational diffusion of TEMPONE in the cytoplasm of Chinese hamster lung cells. Biophys J. 1983 Dec;44(3):405–412. doi: 10.1016/S0006-3495(83)84314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell J. L., Clegg J. S. Cellular and molecular consequences of reduced cell water content. Cryobiology. 1983 Oct;20(5):591–612. doi: 10.1016/0011-2240(83)90048-2. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Tucker J. B. The ground substance of the living cell. Sci Am. 1981 Mar;244(3):56–67. doi: 10.1038/scientificamerican0381-56. [DOI] [PubMed] [Google Scholar]
- Schobert B., Marsh D. Spin label studies on osmotically-induced changes in the aqueous cytoplasm of Phaeodactylum tricornutum. Biochim Biophys Acta. 1982 Feb 10;720(1):87–95. doi: 10.1016/0167-4889(82)90042-8. [DOI] [PubMed] [Google Scholar]
- Wang Y. L., Lanni F., McNeil P. L., Ware B. R., Taylor D. L. Mobility of cytoplasmic and membrane-associated actin in living cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4660–4664. doi: 10.1073/pnas.79.15.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcieszyn J. W., Schlegel R. A., Wu E. S., Jacobson K. A. Diffusion of injected macromolecules within the cytoplasm of living cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4407–4410. doi: 10.1073/pnas.78.7.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol. 1979 Jul;82(1):114–139. doi: 10.1083/jcb.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]


