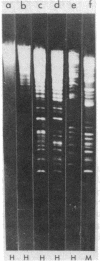
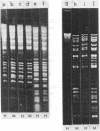
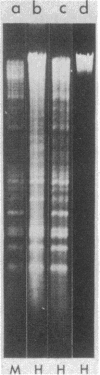
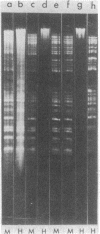
Abstract
We have used inhibitors of methylation to evaluate the proposal that the extent of methylation of chloroplast DNA ( cpDNA ) of the mating type-plus (mt+) parent occurring during gametogenesis in wild-type Chlamydomonas renhardtii is directly correlated with the uniparental transmission of chloroplast genes by this parent [ Sager , R., Grabowy , C. & Sano , H. (1981) Cell 24, 41-47]. As detected by high-pressure liquid chromatography, the methylation of cpDNA was at its lowest level in the vegetative stage; the mt+ cells had a deoxycytidine methylation index (the percentage of deoxycytidine methylated) of 0.5, while the mating type-minus (mt-) index was lower by at least a factor of 3. This basal level of cpDNA methylation increased more than 20-fold after gametogenesis to give a methylation index of 12.1 and 4.3 for mt+ and mt- gametes, respectively. Another striking increase was detected at the 7-hr-zygote stage, resulting in the methylation of nearly half of the total deoxycytidine residues. The extent of zygotic cpDNA methylation was shown to be dependent on the preexisting methylation level of both parental gametic cpDNAs . L-Ethionine and 5-azacytidine effectively inhibited cpDNA methylation during gametogenesis and ensuing zygotic development as shown by both Hpa II/Msp I digestion patterns and HPLC. The transmission of chloroplast genes was analyzed concomitantly with the inhibitor studies. The two inhibitors produced different patterns of inhibition of methylation in mt- and mt+ cells at a given developmental stage. Our overall results demonstrate that the extent of mating type-specific and gamete-specific methylation during gametogenesis is not correlated with the frequency of maternal transmission of chloroplast genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolen P. L., Grant D. M., Swinton D., Boynton J. E., Gillham N. W. Extensive methylation of chloroplast DNA by a nuclear gene mutation does not affect chloroplast gene transmission in chlamydomonas. Cell. 1982 Feb;28(2):335–343. doi: 10.1016/0092-8674(82)90351-8. [DOI] [PubMed] [Google Scholar]
- Burton W. G., Grabowy C. T., Sager R. Role of methylation in the modification and restriction of chloroplast DNA in Chlamydomonas. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1390–1394. doi: 10.1073/pnas.76.3.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang K. S., Kates J. R., Jones R. F., Sueoka N. On the formation of a homogeneous zygotic population in Chlamydomonas reinhardtii. Dev Biol. 1970 Aug;22(4):655–669. doi: 10.1016/0012-1606(70)90174-0. [DOI] [PubMed] [Google Scholar]
- Eves E. M., Chiang K. S. Genetics of Chlamydomonas reinhardtii diploids. I. Isolation and characterization and meiotic segregation pattern of a homozygous diploid. Genetics. 1982 Jan;100(1):35–60. doi: 10.1093/genetics/100.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. M., Gillham N. W., Boynton J. E. Inheritance of chloroplast DNA in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6067–6071. doi: 10.1073/pnas.77.10.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D., Chiang K. S. Physical mapping and characterization of Chlamydomonas mitochondrial DNA molecules: their unique ends, sequence homogeneity, and conservation. Plasmid. 1980 Jul;4(1):82–96. doi: 10.1016/0147-619x(80)90085-2. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Hemimethylated duplex DNAs prepared from 5-azacytidine-treated cells. Nucleic Acids Res. 1981 Jun 25;9(12):2933–2947. doi: 10.1093/nar/9.12.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer H. D., Sager R. Methylation of chloroplast DNAs in the life cycle of Chlamydomonas. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5794–5798. doi: 10.1073/pnas.76.11.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Grabowy C. Differential methylation of chloroplast DNA regulates maternal inheritance in a methylated mutant of Chlamydomonas. Proc Natl Acad Sci U S A. 1983 May;80(10):3025–3029. doi: 10.1073/pnas.80.10.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Grabowy C., Sano H. The mat-1 gene in Chlamydomonas regulates DNA methylation during gametogenesis. Cell. 1981 Apr;24(1):41–47. doi: 10.1016/0092-8674(81)90499-2. [DOI] [PubMed] [Google Scholar]
- Sager R., Lane D. Molecular basis of maternal inheritance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2410–2413. doi: 10.1073/pnas.69.9.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Ramanis Z. Mutations that alter the transmission of chloroplast genes in Chlamydomonas. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4698–4702. doi: 10.1073/pnas.71.12.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Grabowy C., Sager R. Differential activity of DNA methyltransferase in the life cycle of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1981 May;78(5):3118–3122. doi: 10.1073/pnas.78.5.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Sager R. Deoxyribonucleic acid methyltransferase from the eukaryote, Chlamydomonas reinhardi. Eur J Biochem. 1980 Apr;105(3):471–480. doi: 10.1111/j.1432-1033.1980.tb04522.x. [DOI] [PubMed] [Google Scholar]