Abstract
Wnt1 signaling has been implicated as one factor involved in neural crest-derived melanocyte (NC-M) development. Mice deficient for both Wnt1 and Wnt3a have a marked deficiency in trunk neural crest derivatives including NC-Ms. We have used cell lineage-directed gene targeting of Wnt signaling genes to examine the effects of Wnt signaling in mouse neural crest development. Gene expression was directed to cell lineages by infection with subgroup A avian leukosis virus vectors in lines of transgenic mice that express the retrovirus receptor tv-a. Transgenic mice with tva in either nestin-expressing neural precursor cells (line Ntva) or dopachrome tautomerase (DCT)-expressing melanoblasts (line DCTtva) were analyzed. We overstimulated Wnt signaling in two ways: directed gene transfer of Wnt1 to Ntva+ cells and transfer of β-catenin to DCTtva+ NC-M precursor cells. In both methods, NC-M expansion and differentiation were effected. Significant increases were observed in the number of NC-Ms [melanin+ and tyrosinase-related protein 1 (TYRP1)+ cells], the differentiation of melanin− TYRP1+ cells to melanin+ TYRP1+ NC-Ms, and the intensity of pigmentation per NC-M. These data are consistent with Wnt1 signaling being involved in both expansion and differentiation of migrating NC-Ms in the developing mouse embryo. The use of lineage-directed gene targeting will allow the dissection of signaling molecules involved in NC development and is adaptable to other mammalian developmental systems.
Melanocytes are specialized cells that produce melanin-based pigment and are responsible for coloration of the eye, skin, and hair. The majority of melanocytes are found in the epidermis of the skin, stria vascularis of the inner ear, pigmented retinal epithelium, and choroid layer of the eye. Vertebrate melanocytes are derived from two embryonic origins: those in the pigmented retinal epithelium are derived from the neural tube, whereas those in the integument, inner ear, and choroid are derived from the neural crest [neural crest-derived melanocytes (NC-Ms)]. The neural crest is a multipotent population of ectoderm-derived cells that originate around the site and time of dorsal neural tube closure, migrate over defined pathways in the embryos, and differentiate into numerous cell types, including NC-Ms, neurons, and glia of the peripheral nervous system. The process of cell lineage restriction and the path of cell migration are determined by a combination of environmental influences, intercellular interactions, and intrinsic factors.
Wnt signaling is important in many developmental processes including NC-M development. The components of the Wnt signaling pathway have been the subject of recent reviews (1). Wnt genes are a family of secreted, cysteine-rich proteins that act by binding to their receptors in the Frizzled family, ultimately resulting in increased levels of a transcriptional coactivator, β-catenin (βCAT). A complex of βCAT with members of the HMG box-containing transcription factor family, Tcf/Lef, results in altered gene expression, leading to biological effects on cell fate and/or proliferation. Wnt1 and Wnt3a are expressed in the embryonic dorsal neural tube at the time and site of neural crest formation. Marker analysis in embryos deficient for both Wnt1 and Wnt3a demonstrated a marked deficiency of dopachrome tautomerase-positive (DCT+), neural crest-derived melanoblasts, demonstrating the role of these genes in NC-M expansion (2). In zebrafish, injection of βCAT mRNA into a subpopulation of migratory crest also promoted pigment cell formation, but at the expense of neuronal and glia lineages (3). The precise mechanism by which Wnt signaling affects NC-M in mouse development has not been clearly established.
Neural tube explants have been instrumental in the analysis of the signaling factors involved in neural crest development (4–6). In this approach, neural crest cells are studied by dissecting a portion of the neural tube from embryos at 9.5 days postcoitum free from somites and surface ectoderm and culturing in vitro. In culture, a neuroepithelial sheet surrounds the explanted neural tube, and migrating neural crest cells will delaminate from the neural tube and migrate over the epithelial sheet, with some cells continuing to migrate to the periphery of the cultures. Addition of exogenous factors such as Mast cell growth factor and endothelin 3 (EDN3) expand the number of melanoblasts or pigmented NC-Ms, because of effects on NC-M survival, proliferation, and/or differentiation (7–14). A limitation of this mixed-culture system is that the action of genes cannot be restricted to specific lineages. Therefore, it is difficult to directly address whether these genes function in a paracrine or autocrine fashion on NC-Ms. It is also difficult to follow the lineage of individual cells in explant cultures without the use of single-cell culturing. Finally, it is difficult to misexpress genes in a cell-type-directed fashion without generating individual transgenic animals for each construct.
One approach for directing expression of genes to specific cell types is the use of the RCAS-TVA system (15, 16). This system is based on the finding that infection of mammalian cells by a subgroup A avian leukosis virus (the RCAS vector) is not achieved unless the mammalian cell produces the receptor from the avian tv-a gene. Cells or transgenic animals producing the TVA receptor allow infection by RCAS viruses as long as the cells are actively dividing. Once integrated in the mammalian genome, a gene cloned into the RCAS virus is transcribed and translated efficiently from the long terminal repeat; however, infectious viral particles are not formed in mammalian cells. Subsequent spreading to neighboring cells does not occur, making descendants of infected progenitors traceable. Multiple RCAS viruses containing different genes can be used to infect the same cell because of a lack of receptor interference in mammalian cells.
Holland et al. (17) used this system to dissect the molecular pathways underlying glioma formation by targeting genes such as epidermal growth factor receptor to central nervous system progenitor cells in newborn mice. Their study used a Ntva transgenic line of mice. In Ntva+ mice, the tv-a transgene is expressed from a modified nestin promoter, directing transgene expression to central nervous system progenitor cells.
To address some of the embryological limitations of neural crest development in mammalian systems, we have adapted the RCAS-TVA system to allow infection of subpopulations of cells in neural crest cultures. We have used this approach to examine the role of Wnt signaling in the expansion of mouse NC-Ms from neural tube explants. This approach will also have broader applications in dissecting lineage relationships and genetic pathways underlying mammalian development.
Methods
Neural Tube Explant Culture and Infection.
Neural tubes caudal to the otic vesicle and extending to the last somite were dissected from embryonic day 9.5 embryos, leaving somites intact. This tissue was digested with 0.25 mg/ml Dispase (Roche, Molecular Biochemicals) for 5 min at 37°C degrees. Somites and surface ectoderm were removed. Neural tubes were placed in a chamber of a fibronectin-coated, eight-well glass slide containing 0.25 ml of filtered virus supplemented with 10 μm EDN3 (E9137; Sigma). After 24 h, 0.25 ml of growth medium was added. Medium containing 2 μm EDN3 was changed on days 4, 6, 8, 11, and 13 unless otherwise noted. Cultures were grown in 5% CO2 at 37°C for 15 days and fixed in MeOH/DMSO (4:1) or 4% paraformaldehyde. Slides were immunostained and pigmented cells were counted. DMEM contained 10% FBS, glutamine, and antibiotics. DCTTVA neural tubes were infected on days 0, 1, and 4 unless otherwise noted.
Immunohistochemistry.
Indirect immunofluorescence of Wnt1-infected cells was detected by using mouse mAbs to hemagglutinin (HA) (1666606; Roche) or HA.11 (MMS-101P; Covance, Richmond, CA) and anti-mouse fluorescein-labeled secondary antibodies (1814222; Roche) or anti-mouse Texas Red (A-11005; Molecular Probes). NC-Ms were detected with antibodies to TYRP1 [anti-PEP1 (18)] or DCT [anti-Pep8 (18, 19)], a gift from V. Hearing (National Cancer Institute, National Institutes of Health), and by anti-rabbit rhodamine-labeled secondary antibodies (111–295-144; Jackson ImmunoResearch) or anti-rabbit fluorescein (18114257; Roche). Rabbit anti-TVA antibodies [a gift of A. Leavett (University of California), P. Bates (University of Pennsylvania School of Medicine, Philadelphia), and J. Young (Animal Disease and Oncology Laboratory, Agricultural Research Service, United States Department of Agriculture, East Lansing, MI)] were detected by anti-rabbit peroxidase (Vector Laboratories) and developed with diaminobenzidine-peroxidase substrate (D4418; Sigma).
Transgenic Mice.
The Ntva transgenic line constructed and maintained on the FVB/NJ background has been described (17). The transgene designed to direct expression of tv-a (glycosylphosphatidylinositol-linked version of tv-a) behind the Dct/TRP-2 promoter was designed as follows. A NotI fragment derived from pTRP2-lacZ [a gift of Thomas Hornyak (20)] was blunted and cloned into the SmaI site of a pBSK-based plasmid also containing the tv-a cDNA (0.8 kb) (21) and portions of the mouse protamine gene (to supply intron and polyadenylation sequences). This 4.7-kb transgene then was separated from the vector backbone via an EcoRV/XbaI double digest. The purified fragment then was injected into the pronuclei of embryos derived from FVB/NJ mice to generate the DCTtva transgenic mice. All transgenic tv-a embryos and mice were genotyped by PCR (22).
Virus Constructs.
DNA encoding the mouse Wnt1 tagged with an HA epitope at its C terminus (23) was cloned into an ALV-A expression vector, RCAS-Y [a derivative of RCASBP(A) (24) with linker oligonucleotides containing NotI-PmeI-PacI inserted in the ClaI cloning site] linearized with PmeI. The viral DNA construct, RCAS-Wnt1, was used to transfect chicken DF1 cells (a gift from Doug Foster, Avian Disease and Oncology Laboratory, Agriculture Research Services, United States Department of Agriculture, East Lansing, MI), to produce viral stocks. Wnt1 produced by this virus retains its native biological functions as ascertained by a transformation assay (25). A human β-catenin cDNA with a mutation that substitutes Ser for Ala at codon 37 (26) was cloned into the PmeI site of RCAS-Y to generate RCAS-βCATS37A. The S37A point mutation is found in many tumor biopsies and encodes a stabilized form of β-catenin. RCAS-βCATS37AHA is identical to RCAS-βCATS37A except that the encoded βCATS37A is tagged by HA at its C terminus (26). Infections with RCAS-βCATS37A and RCAS-βCATS37AHA led to a potent activation of a Lef/Tcf reporter gene (data not shown).
Whole-Mount in Situ Hybridization.
Antisense RNA probe was generated by transcription with SP6 polymerase of a BamHI-digested plasmid Sp76-TVA by using a digoxigenin-UTP-labeling kit (1277–073; Roche). DCTTVA transgenic and nontransgenic embryos were hybridized as described by ref. 14. Digoxigenin-labeled RNA was bound by antidigoxigenin-conjugated alkaline phosphatase antibodies (1093274; Roche) and detected by nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (18280–016; Life Technologies, Gaithersburg, MD).
Results
Infection of Neural Crest Cells by Using Ntva+ Neural Tube Explants.
The Ntva transgenic mouse line L5 Ntva (17) was used to determine whether RCAS virus infection could be directed to cells present in neural tube explant cultures derived from embryos at 9.5 days postcoitum. Immunohistochemical analyses of 9.5-days postcoitum Ntva+ embryos demonstrated restricted expression of tv-a to neural tube epithelial cells including the dorsal neural tube that contains presumptive neural crest precursors (not shown). Neural tubes derived from matings of hemizygous Ntva transgenic mice with nontransgenic mice were cultured in individual wells in the presence of RCAS-GFP virus overnight and grown for a total of 15 days. Efficient infection of cells occurred in the migratory neural crest, neural tube, and the epithelial sheet in all neural tube explants derived from Ntva+ embryos (n = 6, not shown); between 1% and 75% of the cells were GFP+. As expected, no GFP+ cells were seen in cultures from nontransgenic littermates, demonstrating the lack of a functional retroviral receptor in these embryonically derived cells (n = 6, not shown).
Overexpression of Wnt1 in Ntva+ Cells Results in Expansion of NC-Ms.
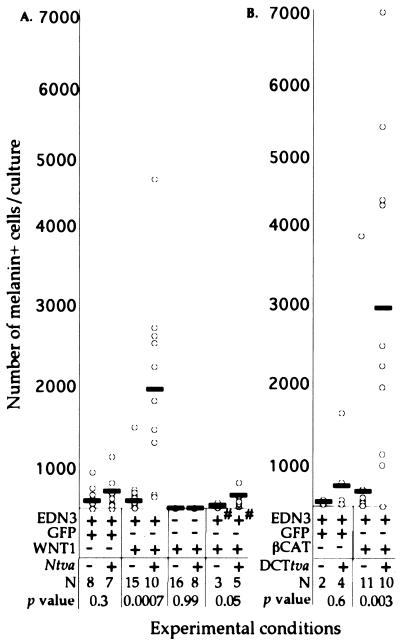
We examined the effects of overexpressed Wnt1 on neural tube explants cultured in the presence of EDN3. Neural tubes isolated from Ntva+ and nontransgenic littermates were cultured in individual wells and infected overnight with RCAS-GFP or RCAS-Wnt1 virus. The occurrence of pigmented cells (melanin+ cells) was measured after 15 days in culture (Figs. 1 and 2A). Infection with RCAS-GFP did not increase the number of pigmented cells (P = 0.3) (Fig. 2A). However, Ntva+ transgenic cultures infected with Wnt1 demonstrated a 7-fold increase in melanin+ cell numbers in comparison with nontransgenic littermates (P < 0.0007) (Figs. 1 C and D and 2A). This result indicated that overexpression of Wnt1 in explant cultures results in increased pigment cell number. Although we observed a variation in the number of infected neural cells, the percentage of infection did not correlate with the number of NC-Ms (R2 = 0.008, data not shown). The Ntva+ cultures were not overtly larger in size than Ntva− cultures, suggesting that Wnt1 was not causing an overall expansion of neural crest cells. More detailed, quantitative analyses need to be done to determine whether other lineages are affected in these cultures.
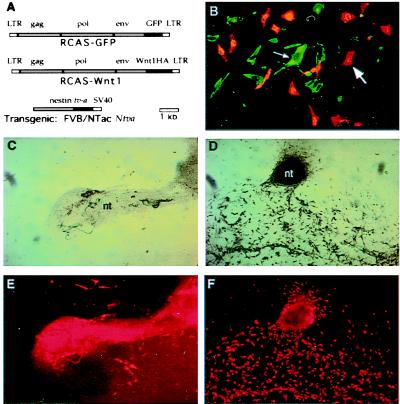
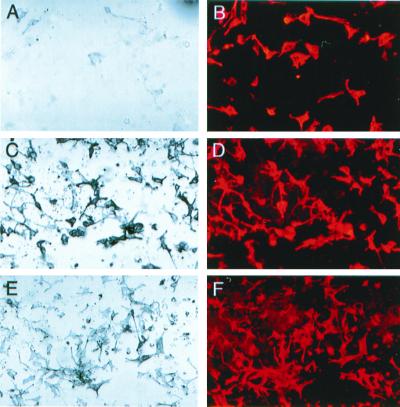
Figure 1.
Infection of neural tube explants with RCAS-Wnt1 retrovirus. (A) Diagram of viral and transgene constructs. RCAS-GFP (Top) and RCAS-Wnt1(C-terminal HA epitope) (Middle) producing retrovirus constructs. (Bottom) The construct used to direct tv-a expression from the modified nestin promoter and enhancer sequences in transgenic mice (17). (B) Photomicrograph of Ntva transgenic neural tube explant infected with RCAS-Wnt1 and immunohistochemically stained for HA epitope representing Wnt1 expression from the retrovirus in green (small arrow) and a TYRP1 epitope representing NC-Ms in red (large arrow). Double staining revealed that Wnt1-expressing cells are a separate population from the pigmented or TYRP1+ cell population. The faint, yellow signals seen in B represent nonspecific binding of the secondary antibody to melanosomes, not coexpression of Wnt1 and TYRP1 in the same cells. (C–F) Photomicrographs of nontransgenic (C and E) and Ntva transgenic (D and F) neural tube explants infected with RCAS-Wnt1. The remaining portion of the neural tube (nt) after 15 days of culture varies between experiments, resulting in the size differences denoted in C and D. Cultures show pigmented (C and D, bright field) and immunohistochemically stained TYRP1 NC-Ms (E and F, red). RCAS-Wnt1-infected cultures show an expansion of pigmented NC-Ms and TYRP1+ NC-Ms. The extensive red in the neural tube is a result of trapping of the secondary antibody in this multicellular structure, not TYRP1 antigenicity. All neural tube explants in this figure were cultured in the presence of EDN3.
Figure 2.
Overexpression of Wnt signaling genes in neural tube explants results in increased numbers of pigmented NC-Ms. Results are shown from neural tube cultures derived from Ntva (A) and DCTtva (B) embryos. (A and B) Graphical representation of the number of pigmented NC-Ms (y axis) resulting from infection of nontransgenic and transgenic neural tube explants with RCAS viruses (x axis). Each open circle represents one neural tube explant culture. The NC-M numbers represent only the melanin+ cells that were not associated with the neural tube. The solid rectangles represent the average number of NC-Ms. For experimental conditions, the + or − represents the addition of EDN3 or the RCAS virus to the cultures, and the +# indicates the addition of EDN3 for only the first 4 days of the 15-day culture. The + or − for Ntva and DCTtva represents neural tubes isolated from transgenic and nontransgenic embryos, respectively. The number of neural tubes per condition is represented as N. The P values are comparisons of the numbers of NC-Ms observed between transgenic and nontransgenic cultures within each experimental condition (Mann–Whitney U analysis).
To confirm that pigmented cells (melanin+) are of the NC-M lineage, explant cultures were immunostained with antibody to a NC-M marker, tyrosinase-related protein 1 (TYRP1), whose expression precedes that of overt melanin deposition during NC-M development (27) (Fig. 1 E and F). Analysis of RCAS-Wnt1-infected Ntva transgenic cultures demonstrated that the majority of expanded melanin+ cells were also TYRP1+ (Fig. 1F), indicating that the expanded cells were NC-M derivatives.
Wnt1 Expansion of NC-Ms Is Endothelin-Dependent.
To determine whether Wnt1 could increase the number of melanin+ cells independent of exogenous endothelin, Ntva transgenic and nontransgenic neural tubes were infected overnight and cultured without the addition of EDN3 for the 15-day culture period. Neither melanin+ cells nor DCT+ cells were detectable in these cultures (Fig. 2A). This result demonstrates that the Wnt1-mediated increase in pigment cell number is dependent on the presence of exogenous EDN3 (Fig. 2A). One possibility is that EDN3 may be needed for melanoblast survival within the first days of culture; and without EDN3, no melanoblasts would be present to respond to Wnt1 signal. To test this, Ntva transgenic and nontransgenic neural tubes were infected with RCAS-Wnt1 overnight and cultured with EDN3 for only the first 4 days and then without the addition of EDN3 for the remainder of the 15-day culture period (Fig. 2A). NC-Ms were present in nontransgenic cultures (average, 50 per culture); however, their numbers were reduced in comparison with nontransgenic cultures that contained EDN3 for the entire 15-day treatment period (average, 286 per culture). Overexpression of Wnt1 in 4-day EDN3-treated cultures allowed a small increase in NC-M number over nontransgenics (P < 0.05), but not to the same extent as when EDN3 was added for the entire culture period (310 per culture in 4-day EDN3 treatment vs. 1,920 per culture in 15-day EDN3 treatment) (Fig. 2A). These data suggest that EDN3 is not needed for the entire culture period to allow NC-M survival and differentiation, but that the Wnt1-induced expansion requires EDN3 for longer than the initial 4-day incubation.
Wnt1-Induced NC-M Expansion Acts in a Paracrine Manner.
Descendants of cells infected with RCAS-Wnt1 can be detected by immunostaining with an antibody to a HA epitope on Wnt1. One to 25% of the cells per neural tube explant demonstrated a positive signal for Wnt1-HA (Fig. 1B). Unexpectedly, immunostaining rarely demonstrated a colocalization of Wnt1-HA with pigment or with TYRP1 antigens (Fig. 1B). Similar results were seen with RCAS-GFP-infected, Ntva+ transgenic neural tube explants cultured in the presence of EDN3 (not shown). It is possible that Wnt1 expression in Ntva+ cells prevents those cells from differentiating into NC-Ms. To test this, Ntva+ cultures were infected overnight with both RCAS-GFP and RCAS-Wnt1 and cultured for 15 days with EDN3. Although extensive infection was observed for both RCAS-Wnt1 and RCAS-GFP, and NC-M expansion occurred, only rarely was GFP colocalized with melanin+ cells. These results suggest that NC-M precursors were not infected efficiently in Ntva transgenic mice under these conditions and that overexpression of Wnt1 does not cause expansion of NC-M by conversion of large numbers of Ntva+ cells along the NC-M lineage. Thus, the NC-M expansion observed occurs by a paracrine effect of Wnt1 acting either directly or indirectly on melanoblasts and/or NC-Ms in these cultures.
Targeted RCAS Infection of NC-M Precursors in DCTtva+ Explants.
To further examine the effect of Wnt signaling on NC-M proliferation and differentiation, we designed an experiment to overexpress a downstream, intracellular effector of Wnt within melanoblasts. A transgenic line of mice was generated that expresses tv-a from the promoter of Dct (28) (line DCTtva). Expression of tv-a in melanoblasts was confirmed by in situ hybridization (Fig. 3B). When neural tube explant cultures prepared from DCTtva+ embryos were infected with RCAS-GFP virus for 4 days and examined 11 days later (total, 15-day culture), production of GFP clearly colocalized with pigmented, differentiated NC-Ms, indicating that NC-M precursors were infected (Fig. 3C). In these cultures we also observed GFP− melanin+ cells, suggesting that precursors for these NC-Ms were not infected, and GFP+ melanin− cells, which could be presumptive melanoblasts that have not yet differentiated into melanin+ NC-Ms. As expected, infection by RCAS-GFP did not overtly alter NC-M number or morphology (Fig. 2B).
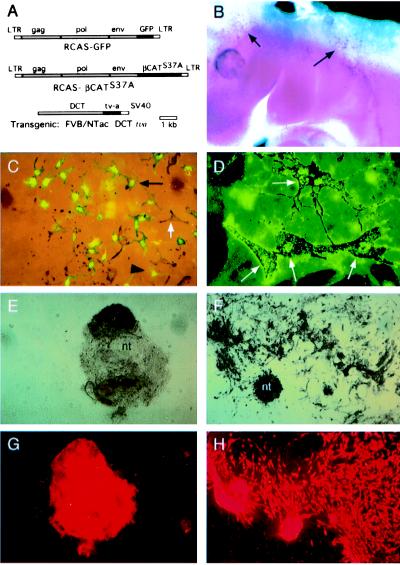
Figure 3.
Lineage-directed infection of RCAS viruses into NC-M precursors using DCTtva transgenic mice. (A) Diagram of RCAS-GFP (Top) and RCAS−βCAT (Middle) viral constructs. The transgene construct (Bottom) used to direct expression of tv-a to melanoblasts in mouse embryos with the Dct promoter is shown. (B) Whole-mount in situ hybridization demonstrates expression of tv-a mRNA in migrating melanoblasts (arrows) of an embryonic day 10.5 DCTtva+ mouse embryo. (C) Photomicrograph demonstrating coexpression of GFP and melanin pigment in NC-Ms derived from neural tube explants of DCTtva embryos infected with RCAS-GFP. GFP expression is seen in both presumptive melanoblasts (large, black arrowhead; unpigmented cell) and pigmented NC-M cells (small, black arrow). A class of melanin+ GFP− NC-Ms also was observed (white arrow), indicating that not all NC-M precursor cells were infected. (D) Colocalization of the HA epitope located on βCAT with pigmented melanocytes (white arrows) in cultures derived from DCTtva+ embryos infected with RCAS-βCAT. (E– H) Photomicrographs of nontransgenic (E and G) and DCTtva transgenic (F and H) neural tube explants infected with βCAT-expressing RCAS virus. Pigmented cultures (E and F, bright field) and immunohistochemically stained TYRP1+ NC-Ms (G and H) are shown. Nontransgenic, uninfected cultures show some melanin+ and TYRP1+ NC-Ms. RCAS-βCAT-infected cultures show an expansion of melanin+ and TYRP1+ NC-Ms.
Overexpression of βCAT in DCTtva+ Cells Results in NC-M Expansion.
Neural tube cultures from DCTtva transgenic matings were infected for 4 days with RCAS expressing a mutant βCAT encoding an activated form (S37A) of this downstream, intracellular effector of Wnt and analyzed as described. As seen with Wnt1 infection of Ntva+ neural tubes, RCAS−βCAT-infected cultures showed a significant increase in the number of melanin+ and TYRP1+ NC-Ms over nontransgenic littermates (Figs. 2B and 3 E–H). Immunostaining for the HA epitope on βCAT demonstrated colocalization with melanin+ cells consistent with βCAT acting in an autocrine fashion in NC-Ms (Fig. 3D). Approximately 95% of the pigmented NC-Ms were HA-positive. These results are consistent with our hypothesis that Wnt1 can act in a paracrine fashion on melanoblasts/NC-Ms to expand the number of NC-Ms in these cultures by signaling through βCAT.
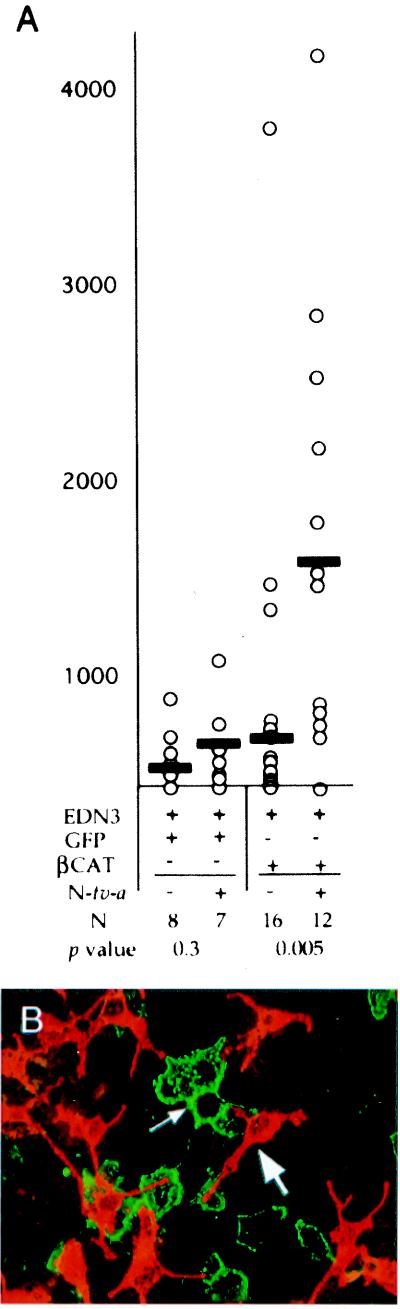
βCAT-expressing Ntva+ cells do not become NC-Ms. In zebrafish, stimulation of Wnt signaling promotes pigment cell formation in subsets of neural crest cells. To explore this in mice, we examined whether overexpression of βCAT in Ntva+ cells could result in NC-M expansion and, if so, whether it occurs by an intrinsic conversion of Ntva+ cells along the NC-M lineage. Neural tubes isolated from Ntva+ and nontransgenic littermates were cultured in individual wells, infected for 4 days with RCAS-βCAT virus, and assayed for the occurrence of melanin+ cells after 15 days in culture, with continuous EDN3 added (Fig. 4A). Infection of Ntva+ cells with RCAS-βCAT resulted in a 2.7-fold increase in NC-Ms (P < 0.005). However, colocalization of βCAT and melanin rarely was observed upon examination of Ntva+ cultures infected with a HA-tagged version of the RCAS-βCAT virus (Fig. 4B). This result suggests that βCAT signaling in Ntva+ cells also can act through a paracrine action to increase NC-Ms in this system. The precise nature and function of this secondary signal require further investigation.
Figure 4.
Infection of Ntva+ cells with βCAT results in NC-M expansion because of a paracrine mode of action. (A) Graphical representation of results from neural tube cultures derived from Ntva embryos. The number of pigmented NC-Ms (y axis) resulting from infection of nontransgenic and transgenic neural tube explants with RCAS viruses (x axis) is shown. Details of the graph are described in the legend for Fig. 2. (B) Photomicrograph of a Ntva+ culture infected with RCAS-βCAT-HA and stained for HA (green, small arrow) and TYRP1 (red, large arrow). Only rarely did colocalization of the two occur in the same cell, demonstrating that the expansion of NC-M is caused by a paracrine effect of βCAT in progenitors of Ntva+ cells.
Wnt Signaling Increases NC-M Differentiation and Pigmentation.
Wnt1 signaling also had an effect on NC-M maturation and differentiation in the neural tube explants. In addition to increasing the number of NC-M cells, these cells were more highly pigmented (Fig. 5) because of an apparent increase in the amount of pigment per cell. This occurred when Wnt1 signaling was stimulated by infection of Ntva with RCAS-Wnt1 or with RCAS-βCAT (Fig. 5 C and E) or by DCTtva infection with RCAS-βCAT (Fig. 3F). In analyzing uninfected cultures, approximately 10% of the TYRP1+ cells were melanin+ (Fig. 5 A and B). However, in the Wnt-stimulated cultures described above, >90% of the TYRP1+ cells were also melanin+ (Figs. 5 C–F and 3 F and H). Taken together, results from this study indicate that Wnt signaling has at least three types of effects on NC-M development in these cultures: an expansion of NC-M numbers, an increased differentiation of TYRP1+ melanin− cells to TYRP1+ melanin+ cells, and an increase in the amount of melanin per melanocyte.
Figure 5.
Stimulation of Wnt signaling increases differentiation and pigmentation of NC-Ms. Bright-field photomicrographs of melanin+ NC-Ms (A, C, and E) and photomicrographs of the same field demonstrating immunohistochemically stained TYRP1+ NC-Ms (B, D, and F) are shown. All are from neural tube explant cultures after treatment for 15 days. Cultures were derived from nontransgenic embryos (A and B), Ntva embryos infected with RCAS-Wnt1 (C and D), or with RCAS-βCAT (E and F). The majority of TYRP1+ cells are pigmented in Wnt-stimulated cultures, whereas most TYRP1+ cells are not overtly pigmented in control cultures (A and B). Pigmented NC-Ms also have an increased amount of pigment within each cell when Wnt1 signaling was stimulated by either Wnt1 (C) or βCAT (E).
Discussion
Several genes and signaling pathways are essential for proper NC-M development and function in the mouse and human (reviewed in refs. 29–31). However, it has been difficult to analyze the function of specific genes without generating knockout animals or individual lines of transgenic animals to express the desired gene product. We have demonstrated that the RCAS-TVA system can be used to generate a functional assay to analyze neural crest development by efficiently introducing genes into subpopulations of neural crest cells in culture.
We have used the RCAS-TVA system to explore the role of Wnt1 signaling in NC-M development. Wnt1 signaling was stimulated in neural tube explant cultures in three ways: overexpression of Wnt1 in descendants of Ntva+ cells, overexpression of activated βCAT in DCT+ melanoblasts/NC-Ms, and overexpression of activated βCAT in Ntva+ cells. Our results demonstrate that increased Wnt1 signaling had at least three effects on NC-Ms in this system: an EDN3-dependent, synergistic expansion of NC-M numbers, an increased differentiation of TYRP1+ melanin− cells to TYRP1+ melanin+ cells, and an increase in pigmentation in NC-Ms. These effects could arise either from a direct action of Wnt1 on NC-Ms and/or an indirect action through an alternative signal provided by an intermediate cell.
Increased Wnt signaling has been associated with increased cell proliferation and tumor formation (including melanoma) in the mouse and humans (32–34). In embryonic development, Wnt signaling also has been associated with decisions regarding cell fate in the fly, worm, and fish. Our results support the former role, with Wnt1 signaling acting on melanoblasts and/or NC-Ms to increase in the number and pigmentation level but not controlling the decision of neural crest precursor cells (Ntva+ cells) to become NC-Ms.
Consistent with our findings, in Wnt1- and Wnt3a-deficient mice, neural crest cells form, yet the numbers of NC-Ms are reduced (2), suggesting that the Wnt pathway is involved in the proliferative expansion of neural crest derivatives in mammals. In chick embryos, neural crest cell formation actually precedes Wnt1 and Wnt3a expression in normal development, and neural crest can form in the absence of Wnt1 and Wnt3a expression (35).
In contrast to the studies in the mouse (2) and chick (35), reporting an association of Wnt signaling with proliferation of neural crest cells, misexpression of Wnt signaling in zebrafish (3) has been proposed to redirect the neural crest lineages formed from precursor cells. Injection of βCAT into a subset of neural crest cells results in the formation of pigment cells at the expense of neurons and glial cells. Because the RCAS-TVA infection system can target expression of genes to specific lineages, we now can use this system to determine whether Wnt can bias the differentiation of neural crest cells in mouse development by generating lines of tva transgenics to direct infection of βCAT to specific lineages.
The synergistic expansion of NC-Ms by Wnt1 was dependent on the addition of EDN3. Endothelin signaling is essential for NC-M development (36). Loss-of-function mutations in EDN3 or its seven-transmembrane receptor EDNRB results in a reduced number of NC-Ms early in development (37, 38), suggesting that, like Wnt signaling, EDN3 is not required for neural crest formation in mice but for expansion of precursor cells. The requirement of EDN3 in neural tube explants suggests that Wnt1 may act synergistically with EDN3 during NC-M development.
Given that the nestin promoter used in these studies directs expression of genes to the embryonic neural tube, including the dorsal aspect that gives rise to the NC, it was surprising that descendants of infected cells from Ntva+ embryos rarely coexpressed NC-M markers. This could be caused by silencing of the RCAS long terminal repeat during differentiation, as has been documented during mouse development (39). However, this is not likely because GFP and βCAT are produced from the RCAS long terminal repeat in NC-Ms derived from DCTtva+ embryos. Therefore, it is more likely that NC-M precursors have a low frequency of infection in Ntva+ cultures. This could occur if NC-M precursors are present but are not dividing rapidly at the time of infection or if the nestin-driven receptor has shut down, conditions nonpermissive for infection by RCAS viruses. Alternatively, a low number of NC-M precursors may be present in the explants at the time of infection. Regardless of the reason, the L5 Ntva line has the added utility of examining genes for paracrine effects on NC-Ms in these cultures and for identifying factors that can direct Ntva+ cells along the NC-M lineage.
Many of the components of the Wnt signaling pathway are members of multigene families. The specific members that are involved in mouse NC-M development and the actions of Wnt signaling are not well understood. One use of this system will be to determine which family members of the Wnt signaling components are involved in NC-M expansion. This system will also allow us to test downstream targets/effectors of Wnt signaling. A few genes recently have been reported to be transcriptionally activated by the Wnt pathway (reviewed in ref. 3), but the relationship of these genes to NC-M development is unknown. The RCAS-TVA system will allow us to explore the relationship of Wnt stimulation and its target genes. In addition, utilization of tv-a-expressing transgenics with mouse NC mutants will allow testing for complementation of spontaneous and engineered mutants (for example, Wnt1−/−; Wnt3a−/− mice) by introducing candidate downstream target/effector genes.
The experiments described in this paper illustrate the potential use of the RCAS/TVA system for manipulating embryonic development with lineage-specific gene transfers. This approach can be adapted to other developmental questions by using appropriate promoters and culturing systems. Although explant cultures are a powerful tool to study NC-M development, they lack the extensive interactions that occur between developing neural crest and embryonic tissues in vivo. We currently are adapting the RCAS-TVA system for use in whole embryo cultures and in vivo gene transfer during mouse embryonic development (K.J.D., Eric Holland, and W.J.P., unpublished results).
Acknowledgments
We thank Eric Holland for the L5 mice and Sp76-TVA plasmid. We thank Harold Varmus, Sandra Orsulic, and members of the Pavan and Varmus labs for insightful discussions and interactions. We thank Arturo Incao for mouse husbandry and genotyping, Lisa Garrett and the National Human Genome Research Institute mouse core facility for generating the transgenic animals used in this study, Amalia Dutra and Derryl Leja for assistance with microphotography and illustrations, Connie Cepko for the RCAS-GFP virus, Doug Foster for the DF1 cells, A. Leavitt, P. Bates, and J. Young for the anti-TVA antibody, and Thomas Hornyak for the Dct promoter plasmid.
Abbreviations
- NC-M
neural crest-derived melanocyte
- RCAS
replication competent avian leukosis virus long terminal repeat splice acceptor
- βCAT
β-catenin
- Ntva
nestin-tva transgene
- DCT
dopachrome tautomerase
- DCTtva
dopachrome tautomerase-tva transgene
- TYRP1
tyrosinase-related protein 1
- EDN3
endothelin 3
- HA
hemagglutinin
References
- 1.Cadigan K M, Nusse R. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 2.Ikeya M, Lee S M, Johnson J E, McMahon A P, Takada S. Nature (London) 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- 3.Dorsky R I, Moon R T, Raible D W. Nature (London) 1998;396:370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- 4.Henion P D, Weston J A. Development. 1997;124:4351–4359. doi: 10.1242/dev.124.21.4351. [DOI] [PubMed] [Google Scholar]
- 5.Stemple D L, Anderson D J. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 6.Stemple D L, Anderson D J. Dev Biol. 1993;159:12–23. doi: 10.1006/dbio.1993.1218. [DOI] [PubMed] [Google Scholar]
- 7.Sieber-Blum M. Biochem Cell Biol. 1998;76:1039–1050. [PubMed] [Google Scholar]
- 8.Opdecamp K, Nakayama A, Nguyen M T, Hodgkinson C A, Pavan W J, Arnheiter H. Development. 1997;124:2377–2386. doi: 10.1242/dev.124.12.2377. [DOI] [PubMed] [Google Scholar]
- 9.Opdecamp K, Kos L, Arnheiter H, Pavan W J. Biochem Cell Biol. 1998;76:1093–1099. [PubMed] [Google Scholar]
- 10.Morrison-Graham K, Weston J A. Dev Biol. 1993;159:346–352. doi: 10.1006/dbio.1993.1246. [DOI] [PubMed] [Google Scholar]
- 11.Lahav R, Dupin E, Lecoin L, Glavieux C, Champeval D, Ziller C, Le Douarin N M. Proc Natl Acad Sci USA. 1998;95:14214–14219. doi: 10.1073/pnas.95.24.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid K, Nishikawa S, Bartlett P F, Murphy M. Dev Biol. 1995;169:568–579. doi: 10.1006/dbio.1995.1170. [DOI] [PubMed] [Google Scholar]
- 13.Reid K, Turnley A M, Maxwell G D, Kurihara Y, Kurihara H, Bartlett P F, Murphy M. Development. 1996;122:3911–3919. doi: 10.1242/dev.122.12.3911. [DOI] [PubMed] [Google Scholar]
- 14.Kos L, Aronzon A, Takayama H, Maina F, Ponzetto C, Merlino G, Pavan W. Pigment Cell Res. 1999;12:13–21. doi: 10.1111/j.1600-0749.1999.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 15.Federspiel M J, Bates P, Young J A, Varmus H E, Hughes S H. Proc Natl Acad Sci USA. 1994;91:11241–11245. doi: 10.1073/pnas.91.23.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher G H, Orsulic S, Holland E, Hively W P, Li Y, Lewis B C, Williams B O, Varmus H E. Oncogene. 1999;18:5253–5260. doi: 10.1038/sj.onc.1203087. [DOI] [PubMed] [Google Scholar]
- 17.Holland E C, Hively W P, DePinho R A, Varmus H E. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsukamoto K, Jackson I J, Urabe K, Montague P M, Hearing V J. EMBO J. 1992;11:519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson I J, Chambers D M, Tsukamoto K, Copeland N G, Gilbert D J, Jenkins N A, Hearing V. EMBO J. 1992;11:527–535. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornyak T J. J Am Acad Dermatol. 1998;39:273–274. doi: 10.1016/s0190-9622(98)70101-x. [DOI] [PubMed] [Google Scholar]
- 21.Bates P, Young J A, Varmus H E. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 22.Holland E C, Varmus H E. Proc Natl Acad Sci USA. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu H, Julius M A, Giarre M, Zheng Z, Brown A M, Kitajewski J. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- 24.Petropoulos C J, Payne W, Salter D W, Hughes S H. J Virol. 1992;66:3391–3397. doi: 10.1128/jvi.66.6.3391-3397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason J O, Kitajewski J, Varmus H E. Mol Biol Cell. 1992;3:521–533. doi: 10.1091/mbc.3.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorn A M, Barish G D, Williams B O, Lavender P, Klymkowsky M W, Varmus H E. Mol Cell. 1999;4:487–498. doi: 10.1016/s1097-2765(00)80200-2. [DOI] [PubMed] [Google Scholar]
- 27.Steel K P, Davidson D R, Jackson I J. Development. 1992;115:1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- 28.Mackenzie M A, Jordan S A, Budd P S, Jackson I J. Dev Biol. 1997;192:99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- 29.Hirobe T, Abe H. Pigment Cell Res. 1999;12:147–163. doi: 10.1111/j.1600-0749.1999.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 30.Erickson C A, Reedy M V. Curr Top Dev Biol. 1998;40:177–209. doi: 10.1016/s0070-2153(08)60367-1. [DOI] [PubMed] [Google Scholar]
- 31.Groves A K, Bronner-Fraser M. Curr Top Dev Biol. 1999;43:221–258. doi: 10.1016/s0070-2153(08)60383-x. [DOI] [PubMed] [Google Scholar]
- 32.Nusse R, Varmus H E. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 33.Rimm D L, Caca K, Hu G, Harrison F B, Fearon E R. Am J Pathol. 1999;154:325–329. doi: 10.1016/s0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 35.Dickinson M E, Selleck M A, McMahon A P, Bronner-Fraser M. Development. 1995;121:2099–2106. doi: 10.1242/dev.121.7.2099. [DOI] [PubMed] [Google Scholar]
- 36.Baynash A G, Hosoda K, Giaid A, Richardson J A, Emoto N, Hammer R E, Yanagisawa M. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 37.Shin M K, Levorse J M, Ingram R S, Tilghman S M. Nature (London) 1999;402:496–501. doi: 10.1038/990040. [DOI] [PubMed] [Google Scholar]
- 38.Pavan W J, Tilghman S M. Proc Natl Acad Sci USA. 1994;91:7159–7163. doi: 10.1073/pnas.91.15.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Federspiel M J, Swing D A, Eagleson B, Reid S W, Hughes S H. Proc Natl Acad Sci USA. 1996;93:4931–4936. doi: 10.1073/pnas.93.10.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]