Abstract
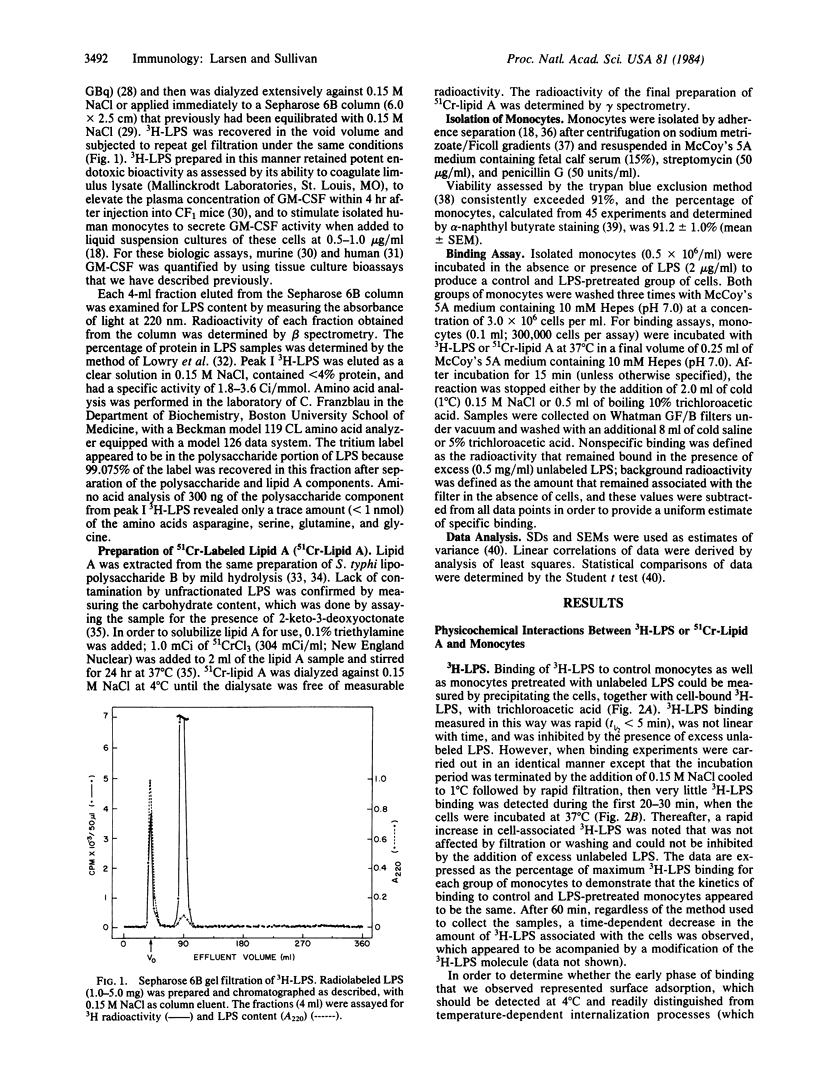
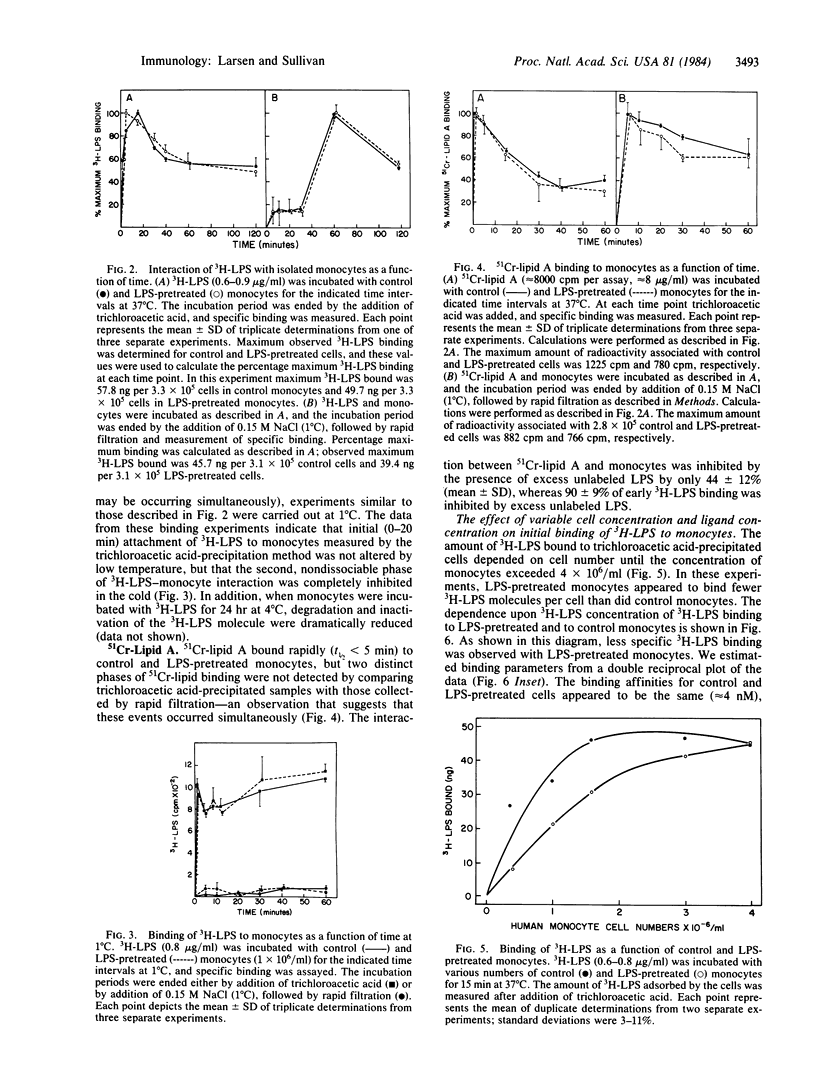
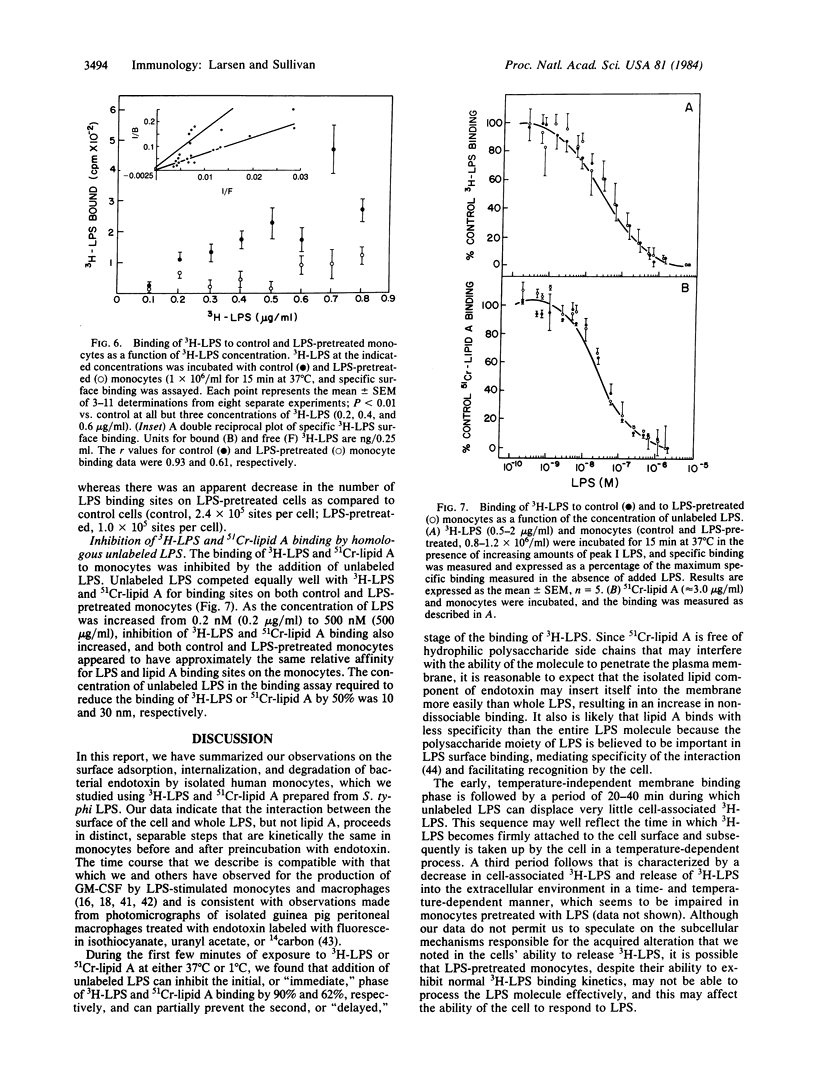
Salmonella typhi endotoxin (lipopolysaccharide, LPS) was labeled with tritium and purified by gel filtration. Using this preparation, we found that binding of 3H-labeled LPS (3H-LPS) to isolated human monocytes consisted of a rapid (t1/2 less than 5 min), reversible, temperature-independent phase of surface adsorption that was followed by a slower (t1/2 greater than 20 min) period of binding that was irreversible and temperature-dependent. The interactions between 3H-LPS and monocytes that we measured were dependent both on the concentration of LPS and the cell number. We observed an apparent decrease in 3H-LPS surface binding after initial treatment of the cells with LPS, which was most likely due to an acquired reduction in the number of sites on the monocyte membrane available for the binding of LPS. Estimates of the parameters of the binding of 3H-LPS were calculated from a double-reciprocal plot (1/bound vs. 1/free) of the surface binding data and suggest that the relative binding affinity (Kd) for 3H-LPS was unchanged after pretreatment of the monocytes with LPS; however, the total number of LPS binding sites appeared to be reduced by this manipulation. The results of competition binding experiments also suggest that the binding affinity for 3H-LPS was the same before and after incubation of the cells with LPS. Lipid A, which we extracted from LPS and labeled with chromium-51, exhibited a binding affinity similar to that of 3H-LPS and, like 3H-LPS, could be displaced from the cells by competing concentrations of unfractionated LPS; however, the kinetics of binding of the two labeled ligands differed considerably. Our results suggest that exposure of monocytes to LPS may alter the ability of these cells to interact with, and consequently respond to, LPS.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte R. N., Galanos C., Pluznik D. H. Lipid A, the active part of bacterial endotoxins in inducing serum colony stimulating activity and proliferation of splenic granulocyte/macrophage progenitor cells. J Cell Physiol. 1976 Jan;87(1):71–78. doi: 10.1002/jcp.1040870110. [DOI] [PubMed] [Google Scholar]
- Apte R. N., Hertogs C. F., Pluznik D. H. Regulation of lipopolysaccharide-induced granulopoiesis and macrophage formation by spleen cells. II. Macrophage-lymphocyte interactions in the process of generation of colony-stimulating factor. J Immunol. 1980 Mar;124(3):1223–1229. [PubMed] [Google Scholar]
- BRAUDE A. I., CAREY F. J., SUTHERLAND D., ZALESKY M. Studies with radioactive endotoxin. I. The use of Cr51 to label endotoxin of Escherichia coli. J Clin Invest. 1955 Jun;34(6):850–857. doi: 10.1172/JCI103140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto D. A., Shands J. W., Jr, Shah D. O. The interaction of bacterial lipopolysaccharide with phospholipid bilayers and monolayers. Biochim Biophys Acta. 1973 Mar 16;298(2):145–157. doi: 10.1016/0005-2736(73)90346-5. [DOI] [PubMed] [Google Scholar]
- Bennett W. E., Cohn Z. A. The isolation and selected properties of blood monocytes. J Exp Med. 1966 Jan 1;123(1):145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham R. B., Castor C. W. The effect of bacterial products on synovial fibroblast function: hypermetabolic changes induced by endotoxin. J Clin Invest. 1972 May;51(5):1186–1194. doi: 10.1172/JCI106912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervenick P. A., LoBuglio A. F. Human blood monocytes: stimulators of granulocyte and mononuclear colony formation in vitro. Science. 1972 Oct 13;178(4057):164–166. doi: 10.1126/science.178.4057.164. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Rothman B., Golde D. W. Effect of endotoxin on the production of colony-stimulating factor by human monocytes and macrophages. J Cell Physiol. 1974 Oct;84(2):193–196. doi: 10.1002/jcp.1040840205. [DOI] [PubMed] [Google Scholar]
- Davies M., Stewart-Tull D. E., Jackson D. M. The binding of lipopolysaccharide from Escherichia coli to mammalian cell membranes and its effect on liposomes. Biochim Biophys Acta. 1978 Apr 4;508(2):260–276. doi: 10.1016/0005-2736(78)90329-2. [DOI] [PubMed] [Google Scholar]
- Dröge W., Lehmann V., Lüderitz O., Westphal O. Structural investigations on the 2-keto-3-deoxyoctonate region of lipopolysaccharides. Eur J Biochem. 1970 May 1;14(1):175–184. doi: 10.1111/j.1432-1033.1970.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Zwiebel R., Cohn Z. A. The pinocytic rate of activated macrophages. J Exp Med. 1975 Nov 1;142(5):1150–1164. doi: 10.1084/jem.142.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin R. J., Wolff S. M. Biology of endotoxin. Annu Rev Med. 1976;27:127–141. doi: 10.1146/annurev.me.27.020176.001015. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Ganguly N. K., Kingham J. G., Lloyd B., Lloyd R. S., Price C. P., Triger D. R., Wright R. Acid hydrolases in monocytes from patients with inflammatory bowel disease, chronic liver disease, and rheumatoid arthritis. Lancet. 1978 May 20;1(8073):1073–1075. doi: 10.1016/s0140-6736(78)90917-0. [DOI] [PubMed] [Google Scholar]
- Gimber P. E., Rafter G. W. The interaction of Escherichia coli endotoxin with leukocytes. Arch Biochem Biophys. 1969 Dec;135(1):14–20. doi: 10.1016/0003-9861(69)90510-4. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Cline M. J. Endotoxin-induced release of colony-stimulating activity in man. Proc Soc Exp Biol Med. 1975 Sep;149(4):845–848. doi: 10.3181/00379727-149-38911. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Cline M. J. Identification of the colony-stimulating cell in human peripheral blood. J Clin Invest. 1972 Nov;51(11):2981–2983. doi: 10.1172/JCI107124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Finley T. N., Cline M. J. Production of colony-stimulating factor by human macrophages. Lancet. 1972 Dec 30;2(7792):1397–1399. doi: 10.1016/s0140-6736(72)92966-2. [DOI] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Chaby R., Cavaillon J. M., Szabó L. Lipopolysaccharide receptor on rabbit peritoneal macrophages. I. Binding characteristics. J Immunol. 1982 May;128(5):1950–1954. [PubMed] [Google Scholar]
- Hammerstrøm J. In vitro influence of endotoxin on human mononuclear phagocyte structure and function. 2. Enhancement of the expression of cytoststic and cytolytic activity of normal and lymphokine-activated monocytes. Acta Pathol Microbiol Scand C. 1979 Dec;87(6):391–399. [PubMed] [Google Scholar]
- Hammerstrøm J., Unsgaard G. In vitro influence of endotoxin on human mononuclear phagocyte structure and function. 1. Depression of protein synthesis, phagocytosis of Candida albicans and induction of cytostatic activity. Acta Pathol Microbiol Scand C. 1979 Dec;87(6):381–389. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Chapman H. A., Jr, Weinberg J. B. Macrophage tumor killing: influence of the local environment. Science. 1977 Jul 15;197(4300):279–282. doi: 10.1126/science.327547. [DOI] [PubMed] [Google Scholar]
- Hämmerling U., Westphal O. Synthesis and use of O-stearoyl polysaccharides in passive hemagglutination and hemolysis. Eur J Biochem. 1967 Mar;1(1):46–50. doi: 10.1007/978-3-662-25813-2_9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Metcalf D. Acute antigen-induced elevation of serum colony stimulating factor (CFS) levels. Immunology. 1971 Sep;21(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Morland B., Kaplan G. Macrophage activation in vivo and in vitro. Exp Cell Res. 1977 Sep;108(2):279–288. doi: 10.1016/s0014-4827(77)80035-9. [DOI] [PubMed] [Google Scholar]
- Quesenberry P., Halperin J., Ryan M., Stohlman F., Jr Tolerance to the granulocyte-releasing and colony-stimulating factor elevating effects of endotoxin. Blood. 1975 Jun;45(6):789–800. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S., Rosenstreich D. L. Macrophage activation for tumor cytotoxicity: control of macrophage tumoricidal capacity by the LPS gene. J Immunol. 1978 Aug;121(2):543–548. [PubMed] [Google Scholar]
- Shaw G. M., Levy P. C., LoBuglio A. F. Human lymphocyte antibody-dependent cell-mediated cytotoxicity (ADCC) toward human red blood cells. Blood. 1978 Oct;52(4):696–705. [PubMed] [Google Scholar]
- Springer G. F., Adye J. C. Endotoxin-binding substances from human leukocytes and platelets. Infect Immun. 1975 Nov;12(5):978–986. doi: 10.1128/iai.12.5.978-986.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staber F. G. Diminished response of granulocyte-macrophage colony-stimulating factor (GM-CSF) in mice after sensitisation with bacterial cell-wall components. Exp Hematol. 1980 Jan;8(1):120–133. [PubMed] [Google Scholar]
- Sullivan R., Gans P. J. Fractionation of human bone marrow cell suspensions in nylon fiber columns: an efficient method for the removal of cells that produce colony stimulating factor (CSF). Exp Hematol. 1980 Sep;8(8):980–987. [PubMed] [Google Scholar]
- Sullivan R., Gans P. J., McCarroll L. A. The synthesis and secretion of granulocyte-monocyte colony-stimulating activity (CSA) by isolated human monocytes: kinetics of the response to bacterial endotoxin. J Immunol. 1983 Feb;130(2):800–807. [PubMed] [Google Scholar]
- Sullivan R., Zuckerman K. S., Quesenberry P. J. The role of colony stimulating activity in modulating murine diffusion chamber granulopoiesis. Br J Haematol. 1980 Mar;44(3):365–374. doi: 10.1111/j.1365-2141.1980.tb05905.x. [DOI] [PubMed] [Google Scholar]
- TENNANT J. R. EVALUATION OF THE TRYPAN BLUE TECHNIQUE FOR DETERMINATION OF CELL VIABILITY. Transplantation. 1964 Nov;2:685–694. doi: 10.1097/00007890-196411000-00001. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Dean J., Eilat D., Lorenz P. E., Schechter A. N. Tritium labeling of proteins to high specific radioactivity by reduction methylation. J Biol Chem. 1980 Sep 25;255(18):8842–8847. [PubMed] [Google Scholar]
- Tomasulo P. A., March S. C., Levin Tritiation of endotoxin. Biochim Biophys Acta. 1975 Aug 19;400(2):399–406. doi: 10.1016/0005-2795(75)90195-6. [DOI] [PubMed] [Google Scholar]
- VOGEL R. A. Role of lipid in hemagglutination by Candida albicans and Saccharomyces cerevisiae. Proc Soc Exp Biol Med. 1957 Feb;94(2):279–283. doi: 10.3181/00379727-94-22920. [DOI] [PubMed] [Google Scholar]



