Abstract
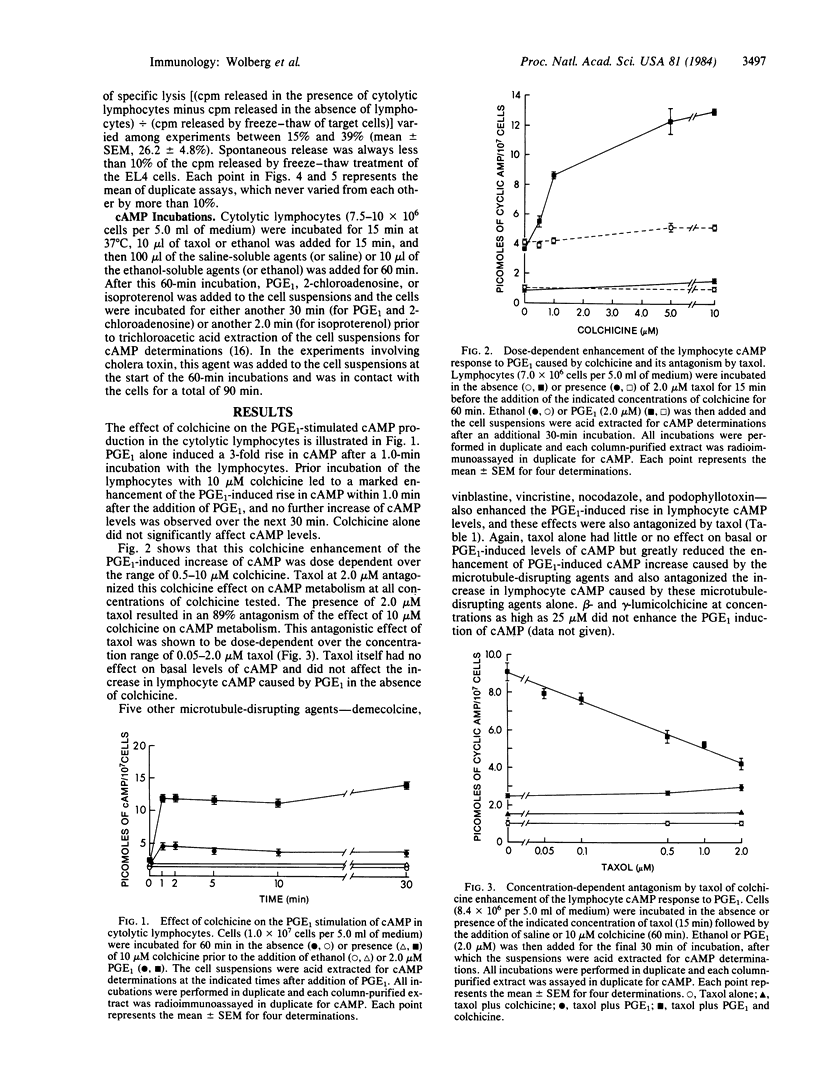
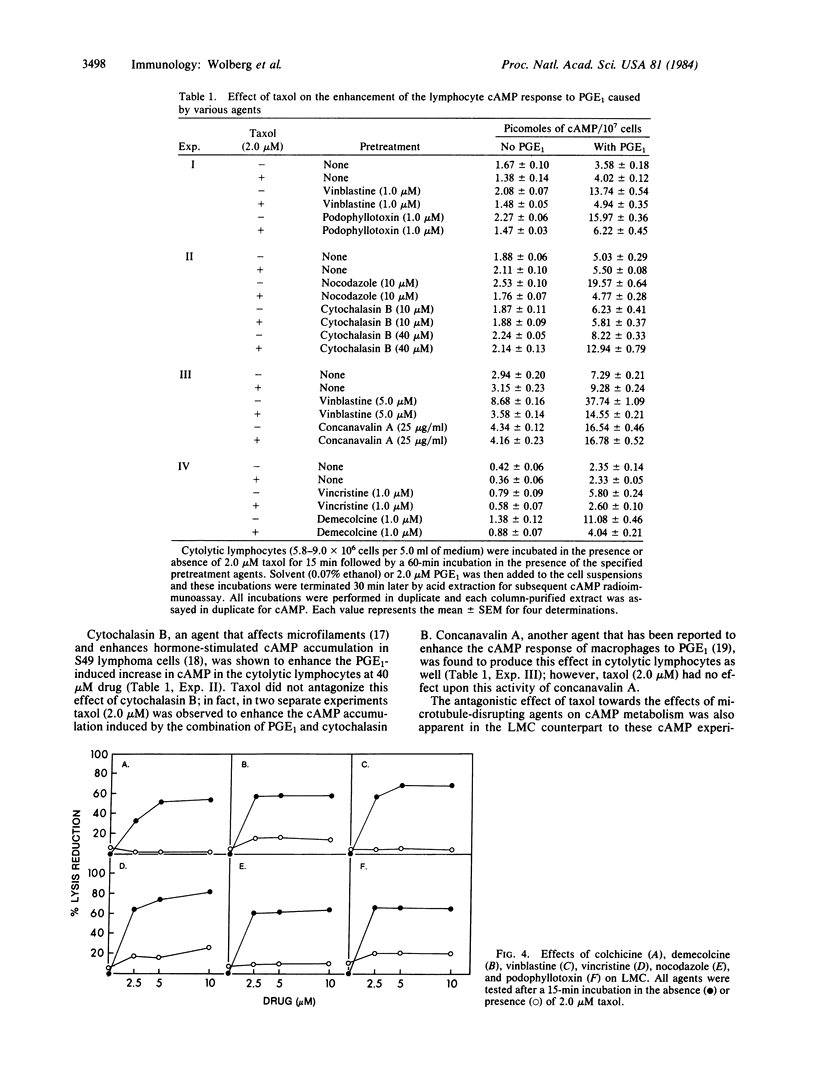
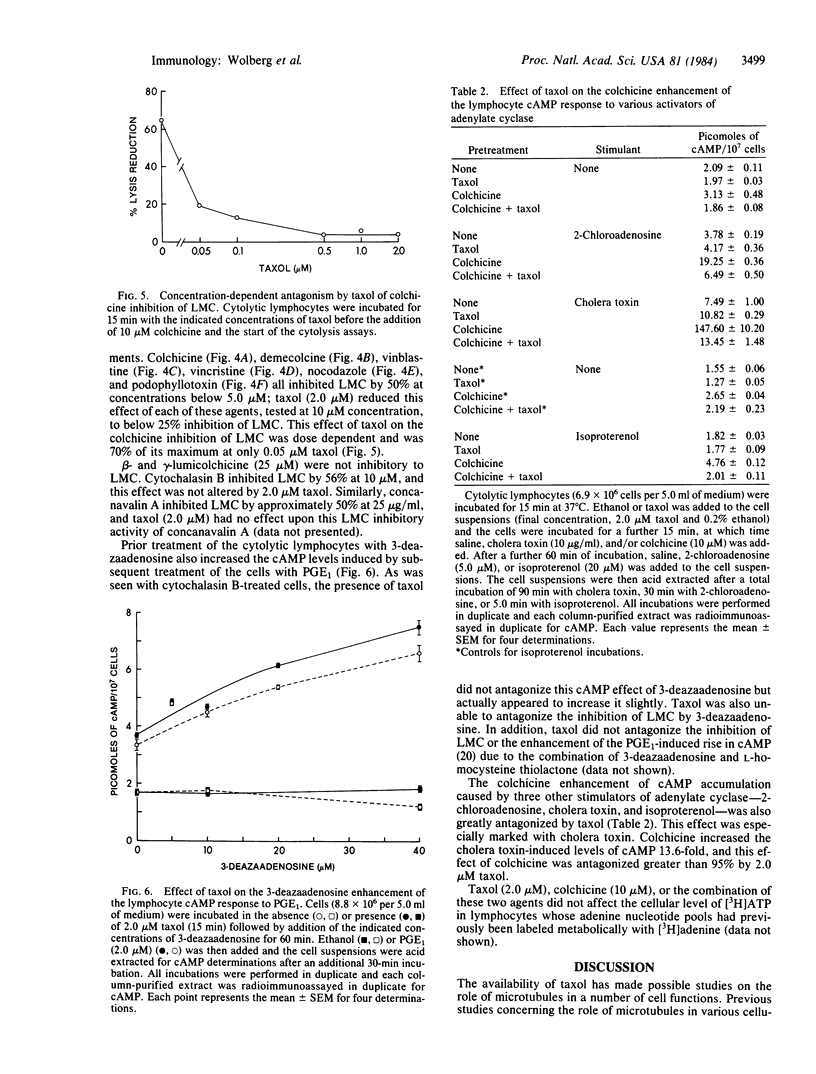
Several microtubule-disrupting agents (colchicine, demecolcine, vinblastine, vincristine, podophyllotoxin, and nocodazole) have been shown to inhibit lymphocyte-mediated cytolysis. These agents also enhanced the prostaglandin E1-induced rise in cAMP levels in these cytolytic lymphocytes. Taxol, a natural product alkaloid that has been shown to enhance microtubule polymerization and to stabilize microtubules, antagonized both of these effects of the microtubule-disrupting agents in the cytolytic lymphocytes. Taxol also antagonized the enhancement of cAMP increases by colchicine in lymphocytes stimulated by 2-chloroadenosine, isoproterenol, and cholera toxin. The enhancement of the prostaglandin E1-induced cAMP response caused by treatment of the lymphocytes with either cytochalasin B or 3-deazaadenosine in the presence or absence of L-homocysteine was not antagonized by taxol. Taxol, colchicine, or the combination of these two agents did not affect ATP levels in cytolytic lymphocytes. These results support a modulatory role for microtubules in both the cytolytic process and the production of cAMP in these lymphocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuthbert J. A., Shay J. W. Microtubules and lymphocyte responses: effect of colchicine and taxol on mitogen-induced human lymphocyte activation and proliferation. J Cell Physiol. 1983 Aug;116(2):127–134. doi: 10.1002/jcp.1041160202. [DOI] [PubMed] [Google Scholar]
- DoKhac L., Tanfin Z., Harbon S. Differential role of microtubules in the control of prostaglandin E2 and beta-adrenergic stimulation of cyclic AMP accumulation in the rat myometrium. Biochem Pharmacol. 1983 Sep 1;32(17):2535–2541. doi: 10.1016/0006-2952(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Duncan G. S., Wolberg G., Schmitges C. J., Deeprose R. D., Zimmerman T. P. Inhibition of lymphocyte-mediated cytolysis and cyclic AMP phosphodiesterase by erythro-9-(2-hydroxy-3-nonyl)adenine. J Immunopharmacol. 1982;4(1-2):79–100. doi: 10.3109/08923978209031077. [DOI] [PubMed] [Google Scholar]
- Furcht L. T., Scott E. Effect of vinblastine sulfate, colchicine and lumicolchicine on membrane organization of normal and transformed cells. Exp Cell Res. 1975 Dec;96(2):271–282. doi: 10.1016/0014-4827(75)90257-8. [DOI] [PubMed] [Google Scholar]
- Gemsa D., Steggemann L., Till G., Resch K. Enhancement of the PGE1 response of macrophages by concanavalin A and colchicine. J Immunol. 1977 Aug;119(2):524–529. [PubMed] [Google Scholar]
- Greene W. C., Parker C. M., Parker C. W. Colchicine-sensitive structures and lymphocyte activation. J Immunol. 1976 Sep;117(3):1015–1022. [PubMed] [Google Scholar]
- Henney C. S., Bubbers J. E. Antigen-T lymphocyte interactions: inhibition by cytochalasin B. J Immunol. 1973 Jul;111(1):85–90. [PubMed] [Google Scholar]
- Horwitz S. B., Chia G. H., Harracksingh C., Orlow S., Pifko-Hirst S., Schneck J., Sorbara L., Speaker M., Wilk E. W., Rosen O. M. Trifluoperazine inhibits phagocytosis in a macrophagelike cultured cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):798–802. doi: 10.1083/jcb.91.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Hii C. S., Shaikh S., Tyhurst M. Effects of taxol and nocodazole on insulin secretion from isolated rat islets of Langerhans. Biosci Rep. 1982 Oct;2(10):795–801. doi: 10.1007/BF01114939. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Koachman A. M. Cytochalasin B enhances hormone and cholera toxin-stimulated cyclic AMP accumulation in S49 lymphoma cells. J Biol Chem. 1982 Aug 25;257(16):9717–9723. [PubMed] [Google Scholar]
- Katz P., Zaytoun A. M., Lee J. H., Jr Mechanisms of human cell-mediated cytotoxicity. III. Dependence of natural killing on microtubule and microfilament integrity. J Immunol. 1982 Dec;129(6):2816–2825. [PubMed] [Google Scholar]
- Landowne D., Larsen J. B., Taylor K. T. Colchicine alters the nerve birefringence response. Science. 1983 May 27;220(4600):953–954. doi: 10.1126/science.6302838. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Nath J., Flavin M., Schiffmann E. Stimulation of tubulin tyrosinolation in rabbit leukocytes evoked by the chemoattractant formyl-methionyl-leucyl-phenylalanine. J Cell Biol. 1981 Oct;91(1):232–239. doi: 10.1083/jcb.91.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Ikehara Y. Taxol, a potent promoter of microtubule assembly, inhibits secretion of plasma proteins in cultured rat hepatocytes. Biochem Biophys Res Commun. 1982 Jul 30;107(2):561–567. doi: 10.1016/0006-291x(82)91528-5. [DOI] [PubMed] [Google Scholar]
- Paatero G. I., Brown D. L. Effects of taxol on microtubule organization and on capping of surface immunoglobulin in mouse splenic lymphocytes. Cell Biol Int Rep. 1982 Nov;6(11):1033–1040. doi: 10.1016/0309-1651(82)90019-4. [DOI] [PubMed] [Google Scholar]
- Roberts R. L., Nath J., Friedman M. M., Gallin J. I. Effects of taxol on human neutrophils. J Immunol. 1982 Nov;129(5):2134–2141. [PubMed] [Google Scholar]
- Rudolph S. A., Greengard P., Malawista S. E. Effects of colchicine on cyclic AMP levels in human leukocytes. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3404–3408. doi: 10.1073/pnas.74.8.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani M. C., Taylor H. L., Wall M. E., Coggon P., McPhail A. T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971 May 5;93(9):2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- White J. G., Rao G. H. Effects of a microtubule stabilizing agent on the response of platelets to vincristine. Blood. 1982 Aug;60(2):474–483. [PubMed] [Google Scholar]
- Wolberg G., Hümstra K., Burge J. J., Singler R. C. Reversible inhibition of lymphocyte mediated cytolysis by dimethyl sulfoxide (DMSO). J Immunol. 1973 Nov;111(5):1435–1443. [PubMed] [Google Scholar]
- Zimmerman T. P., Rideout J. L., Wolberg G., Duncan G. S., Elion G. B. 2-Fluoroadenosine 3':5'-monophosphate. A metabolite of 2-fluoroadenosine in mouse cytotoxic lymphocytes. J Biol Chem. 1976 Nov 10;251(21):6757–6766. [PubMed] [Google Scholar]
- Zimmerman T. P., Schmitges C. J., Wolberg G., Deeprose R. D., Duncan G. S., Cuatrecasas P., Elion G. B. Modulation of cyclic AMP metabolism by S-adenosylhomocysteine and S-3-deazaadenosylhomocysteine in mouse lymphocytes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5639–5643. doi: 10.1073/pnas.77.10.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. P., Wolberg G., Duncan G. S. Inhibition of lymphocyte-mediated cytolysis by 3-deazaadenosine: evidence for a methylation reaction essential to cytolysis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6220–6224. doi: 10.1073/pnas.75.12.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]



