Abstract
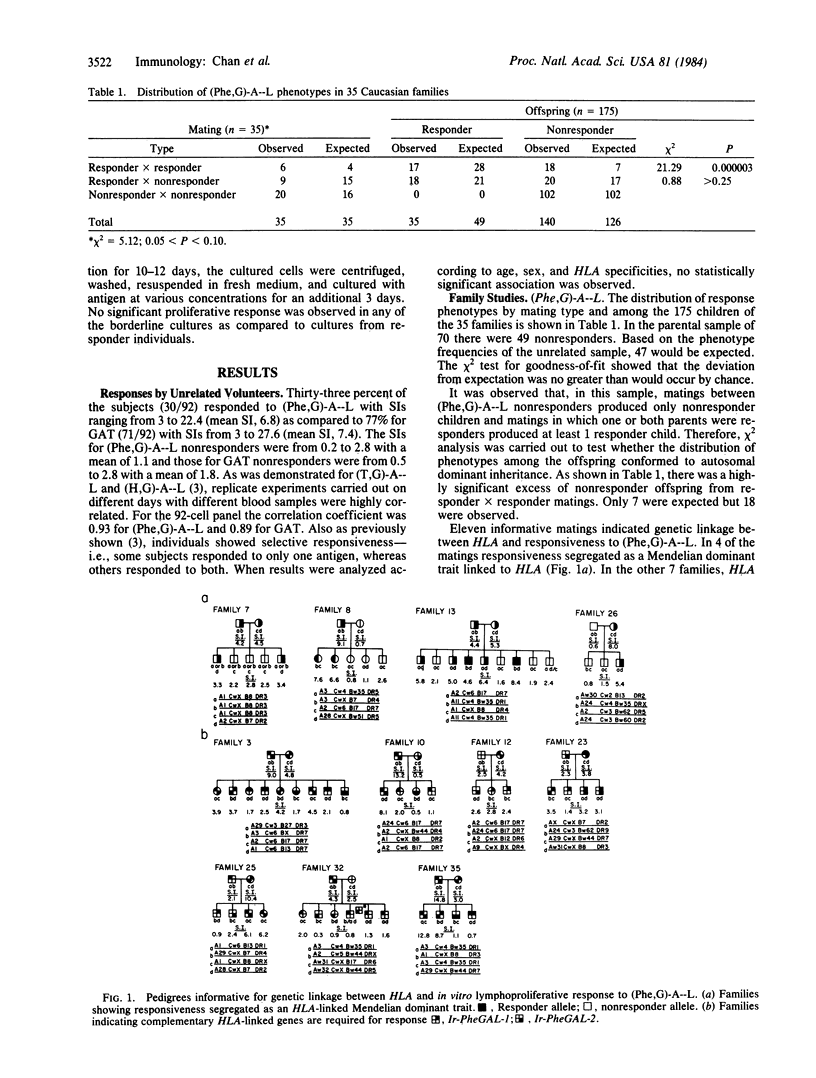
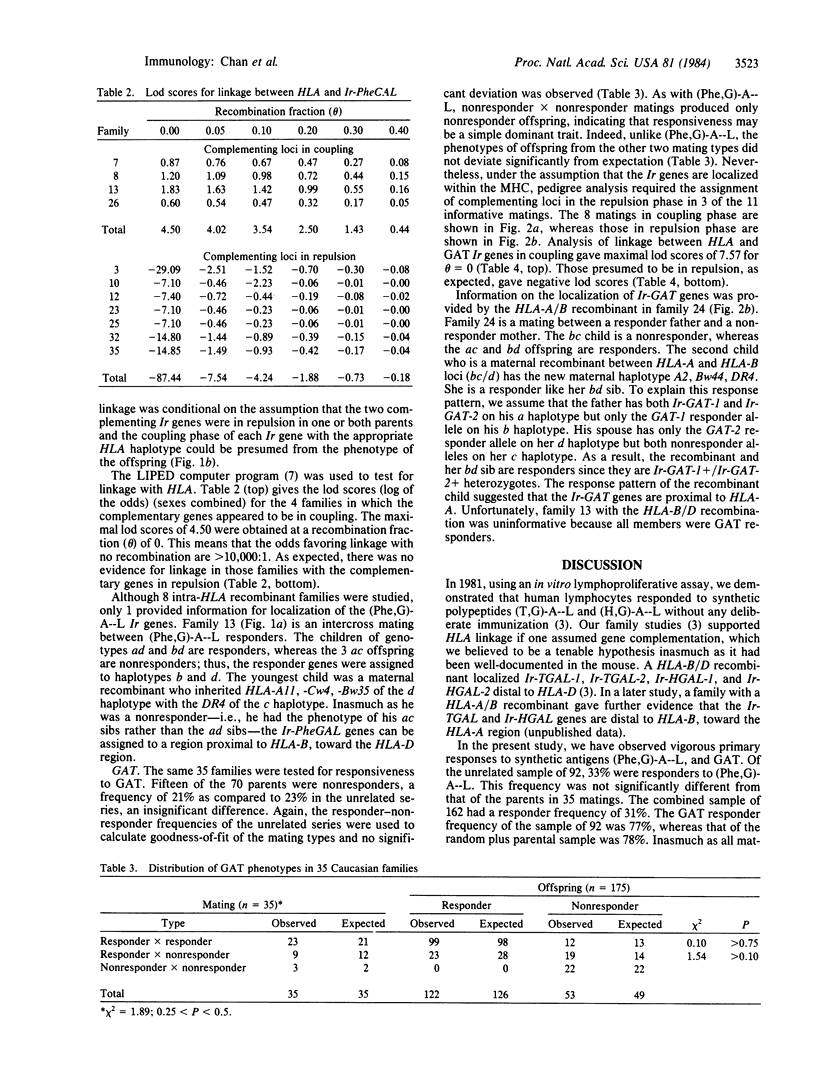
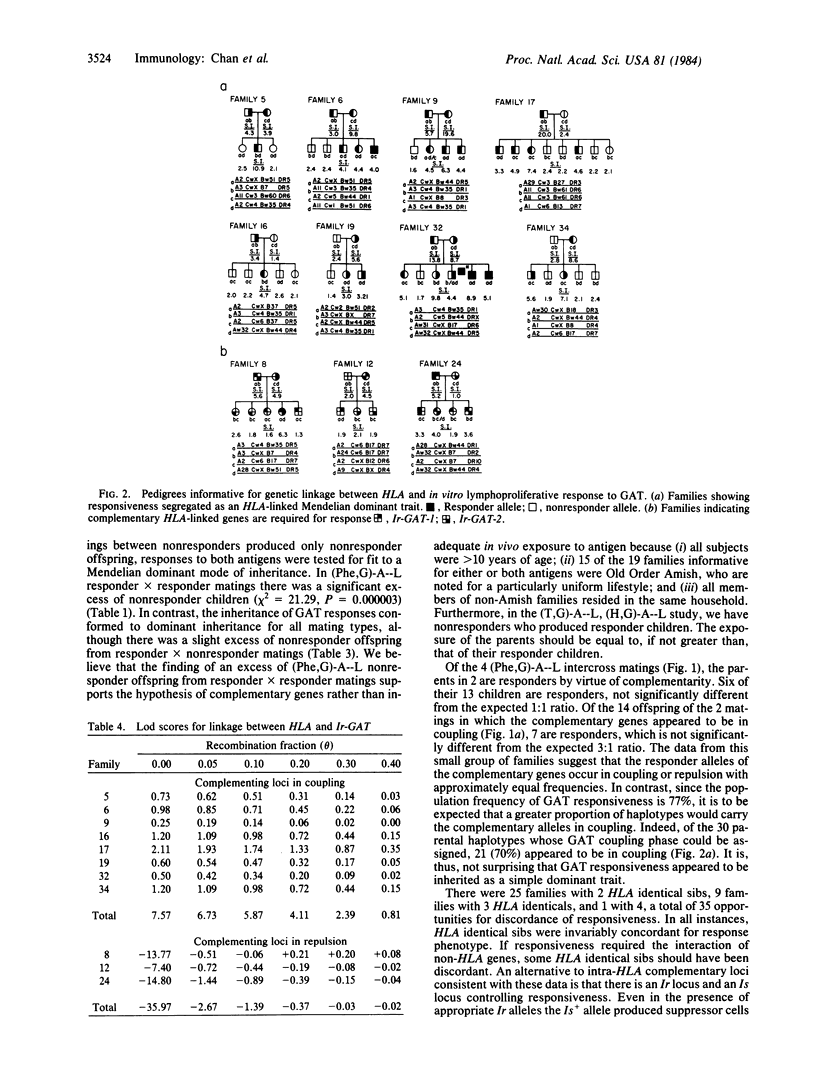
Vigorous lymphoproliferative responses to synthetic polypeptides poly(L-phenylalanine, L-glutamic acid)-poly(DL-alanine)--poly(L-lysine) [( Phe,G)-A--L], and L-glutamic acid, L-alanine, L-tyrosine (60:30:10) (GAT) were observed in cells from 92 unrelated subjects. Thirty-three percent responded to (Phe,G)-A--L and 77% to GAT. No HLA association was observed with responses to these two antigens. Family studies indicated that two complementary immune response (Ir) genes are required for response to each antigen. Eleven matings were informative for linkage analysis between HLA and these Ir genes. Families in which the complementary genes are in coupling gave maximal lod scores (log of the odds) of 4.50 for (Phe,G)-A--L and 7.57 for GAT for 0 = 0. In a HLA-B/D recombinant family, the Ir- PheGAL genes are mapped towards the HLA-D region. The localization of Ir-GAT genes close to HLA-B was provided by a HLA-A/B recombinant.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B., Katz D. H. The histocompatibility-linked immune response genes. Adv Cancer Res. 1975;21:121–173. doi: 10.1016/s0065-230x(08)60972-0. [DOI] [PubMed] [Google Scholar]
- Eijsvoogel V. P., van Rood J. J., Du Toit E. D., Schellekens P. T. [Position of a locus determining mixed lymphocyte reaction distinct from the known HL-A loci]. Eur J Immunol. 1972 Oct;2(5):413–418. doi: 10.1002/eji.1830020506. [DOI] [PubMed] [Google Scholar]
- Hsu S. H., Chan M. M., Bias W. B. Genetic control of major histocompatibility complex-linked immune responses to synthetic polypeptides in man. Proc Natl Acad Sci U S A. 1981 Jan;78(1):440–444. doi: 10.1073/pnas.78.1.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. G., Bona C. Proteases as mitogens. The Effect of trypsin and pronase on mouse and human lymphocytes. Exp Cell Res. 1974 Oct;88(2):388–394. doi: 10.1016/0014-4827(74)90257-2. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Benacerraf B. Genetic control of specific immune responses. Adv Immunol. 1969;11:31–74. doi: 10.1016/s0065-2776(08)60477-0. [DOI] [PubMed] [Google Scholar]
- Ott J. Estimation of the recombination fraction in human pedigrees: efficient computation of the likelihood for human linkage studies. Am J Hum Genet. 1974 Sep;26(5):588–597. [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Pollack M. S., Payne S. M., Johnson A. H. HLA-linked B cell alloantigens of a new segregant series: population and family studies of the SB antigens. Hum Immunol. 1980 Sep;1(2):177–185. doi: 10.1016/0198-8859(80)90104-4. [DOI] [PubMed] [Google Scholar]



