Abstract
Purpose : To investigate a possible association between testicular cancer or undescended testis and Y microdeletions.
Methods : It was designed as a retrospective clinical study. A total of 225 men with testicular cancer or undescended testis were included to study. Fertile men (n = 200) were investigated as a control. Genomic DNA, which was extracted from blood samples were investigated with a fluorescent multiplex PCR protocol for screening for Y microdeletions
Results : A single STS missing was found in eight men; one from the control group (sY153), seven from the patients group. The positive cases showed a single STS missing of marker sY153 and sY139 in testicular cancer (6/185) and undescended testis (1/40) patients, respectively.
Conclusions : Since no contiguous, real Y microdeletions were found in the study population, it seems that Y microdeletions are not a likely common etiological cause of poor spermatogenesis in testicular cancer and undescended testis. However, it remains to be determined whether men having a single STS missing have a risk of developing testis cancer or having undescended testis.
KEY WORDS: Capillary electrophoresis, multiplex PCR, oligo-azoospermia, testis cancer, undescended testis, Y microdeletions.
INTRODUCTION
Testicular malignancies are the most common solid tumors in men 15–35 years of age (1). The incidence of testicular cancer has been increasing over the past decades. Oligozoospermia, testicular atrophy, and a history of undescended testis might be factors associated with increased risk of testicular cancer (1,2).
Several previous reports discuss infertility as the presenting symptom of testicular cancer and show a high incidence—ranging from 0.4 to 1.6%—of carcinoma in situ in infertile men (3). About 10% of testicular cancers occur in an undescended testis (2). Men with an undescended testis have impaired spermatogenesis (azoospermia or oligozoospermia in 50–70%), and infertility (2,4).
Recently, genetic etiologies such as karyotypeabnormalities and microdeletions on the Y chromosome have been shown to play an increasingly important role in impaired spermatogenesis. Three regions that are responsible for spermatogenetic defects are known as “azoospermia factor” (AZF) a, b, and c region, from proximal to distal Yq11 (5). It is believed that microdeletions on the Y chromosome are the cause of 10–15% of cases with idiopathic azoospermia or severe oligozoospermia. However, higher frequencies were found when evaluating the most severe idiopathic primary testiculopathies (up to 55%) (5).
A striking association seems to exist between impaired spermatogenesis, testicular cancer, and undescended testis, which may point to a possible common etiology. Since a genetic etiology related to Y microdeletions has been shown to be a cause of male infertility, we find it important to elucidate the role of Y microdeletions in undescended testis or testicular cancer.
Thus, in this study, we investigate a possible association between Y microdeletions and testicular cancer or undescended testis to clarify whether Y microdeletions could be a causal factor in these cases.
MATERIALS AND METHODS
Patients Selection
The study was retrospective. A total of 185 men with testicular cancer and 40 men with undescended testis accepted to participate in this study. Written consent was given according to the study protocol, which was approved by the Regional Ethics Committee. Fertile men (n = 200), who had fathered a minimum four children spontaneously, were included in the study as a control.
Genomic DNA was extracted as described previously (6). Sperm analysis was optional.
Screening for Y Microdeletions with Fluorescent Multiplex PCR and Capillary Electrophoresis
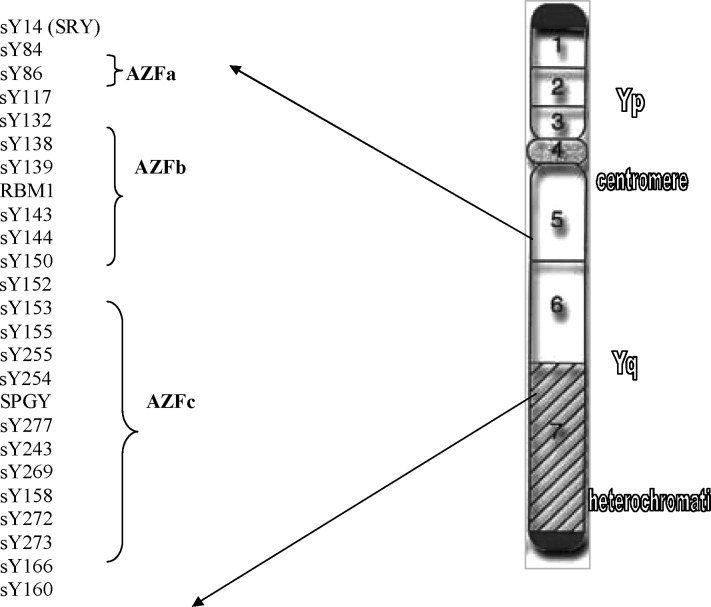
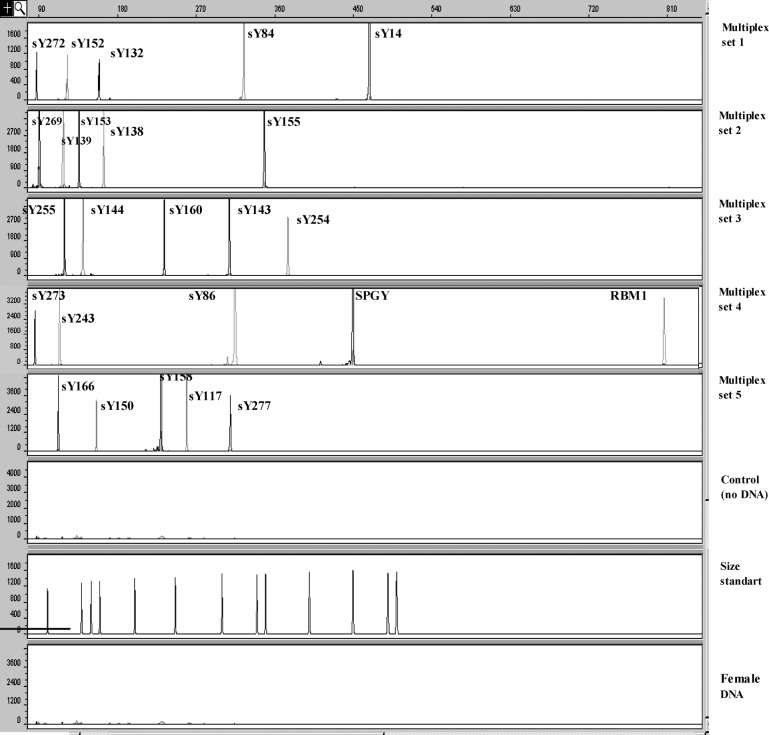
A fluorescent multiplex PCR protocol was performed for screening for Y microdeletions as described previously (7). Briefly, 25 sequence-tagged sites (STSs) covering the AZFa, b, and c regions, which were amplified in five multiplex PCR sets (Fig. 1). One of the primers of each pair was labeled with a fluorescent tag, either FAM (carboxyfluorescin) or HEX (hexachloro-6-carboxyfluorescein) to the 5′-end. Analysis of the obtained PCR products was performed using capillary electrophoresis (ABI Prism 3100 Genetic Analyzer), which has been described previously (7). The results of multiplex PCR combined with capillary electrophoresis for screening for Y microdeletions in a fertile man are presented in Fig. 2.
Fig. 1.
Diagram of interval 6 of the Y chromosome. STSs used in the present study for screening for Y microdeletions are indicated.
Fig. 2.
Twenty-five peaks representing 25 amplified DNA fragments from AZF regions by the fluorescent-multiplex PCR are presented in five electropherograms. The PCR products labeled with FAM and HEX dye are represented as gray- and black-colored peaks, respectively.
Internal quality control for the diagnosis of Y microdeletions was performed as described previously (7).
RESULTS
The age of the 40 men with undescended testis and of the 185 men with testicular cancer ranged from 21 to 40 and from 22 to 61 years, respectively (mean age 32 and 37, respectively). All participants were Danish.
Sperm analysis was performed in 12 men of the 40 men with undescended testis. Two were azoospermic, 3 oligozoospermic, 7 normozoospermic with sperm count varying from 0 to 65 million, with a mean of 21 million. Semen was available for analysis in 11 of the 185 men with testicular cancer. Five were azoospermic, four oligozoospermic, and two normozoospermic with sperm counts varying from 0 to 150 million, with a mean of 23 million.
Six (15%) and 44 (23.7%) men with undescended testis and testicular cancer, respectively, were involuntary childless. Twenty-four men with testicular cancer had a history of undescended testis previously (12.9%).
All 185 men studied had unilateral testicular cancer. None of these had carcinoma in situ in the contralateral testis. Histology examination showed that 86 were seminomas and 99 non-seminoma germ cell tumors.
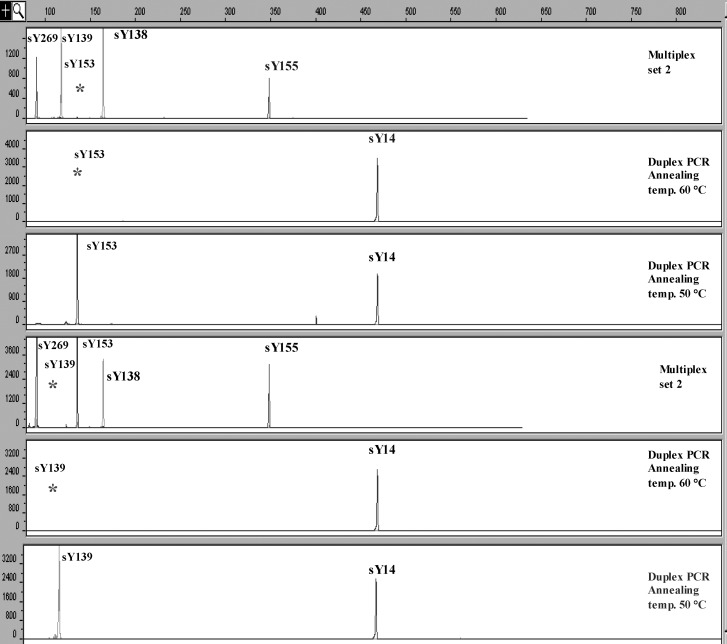
A single STS missing was found in seven men from the patient group and one from the control group. These cases showed a single STS missing for sY153 in six cases of testicular cancer (6/185, 3.2%) and a single STS missing for sY139 in one case of undescended testis (1/40, 2.5%). One man from the control group also had a single STS missing with sY153 (1/200, 0.5%). These results were confirmed by performing duplex PCR with sY14 (SRY) and sY153 or sY139, respectively. The other STS markers that cover the same region were present in all eight cases. When performing duplex PCR with 50°C annealing temperature in all cases (in stead of 60°C), we demonstrated presence of both sY153 and sY139 products of the expected size (Fig. 3), which indicates that the missing signals represent a sequence variation in the primer binding site, and not a real deletions.
Fig. 3.
A single STS missing for sY153 and sY139 in testicular cancer and undescended testis patients, respectively, was found in multiplex PCR, which is presented in electropherograms 1 and 4. These results were confirmed by performing duplex PCR with sY14 (SRY) and sY153 or sY139, retrospectively (electropherograms 2 and 5). By performing duplex PCR with 50°C annealing temperature (instead of 60°C), the presence of both sY153 and sY139 products of the expected size was demonstrated (electropherograms 3 and 6).
DISCUSSION
A high frequency of Y microdeletions has been reported (up to 55%) in men having oligozoospermia or azoospermia (5). An association between testicular cancer and male infertility has been studied and reported in many studies. Furthermore, 60% of men with testicular cancer have impaired spermatogenesis (3). Therefore, we anticipated a high frequency of Y microdeletions in testicular cancer patients. However, of 185 testicular cancer patients, we found only single STSs missing (sY153) in six men. Furthermore, the same STS (sY153) was found to be missing in 1 of 200 fertile men. We believe that these are sequence variants, and not real Y microdeletions due to the presence of PCR products of these STSs are demonstrated by using lower annealing temperature in the PCR.
Two recent studies have investigated the association between Y microdeletions and testicular cancer (8,9). In the first study, a high frequency of AZF deletions involving 1–8 STSs were reported in normal and/or tumor samples from 13 out of 17 Finnish men with a history of testicular cancer (76.4%). Interestingly, none of the deletions represented a contiguous pattern with more than two STSs and they were not confirmed by Southern blot. Therefore, it is difficult to conclude that these are real Y microdeletions related to spermatogenic failure. In the second study, AZF microdeletion was screened in 160 Danish patients with testicular germ cell neoplasia looking for a possible association between AZF microdeletions, infertility, and germ cell neoplasia. In this study, no microdeletion was found in 160 men with testicular germ cell neoplasia—in accordance with the findings of the present study. Interestingly, the authors of the last study opened a new discussion by suggesting that AZF deletions might cause an early depletion of germ cells and effectively decrease the chance of overt germ cell tumors to develop, since no carcinoma in situ (CIS) cells have been reported in testicular biopsies from infertile men with AZF microdeletions.
A few studies have reported on a possible relationship between Y microdeletions and undescended testis with varying results (10,11). Foresta et al. reported Y microdeletions in 11 of 40 (27.5%) men with unilaterally undescended testis (11). On the other hand, Fagerli et al. found no Y microdeletions in a total of 42 men diagnosed with undescended testis, who had normal, decreased, or absent sperm on semen analysis (10). These controversial results could be due to the several factors including the low number of patients studied, patient selection criteria, methodological aspects, and geographical or ethnical differences. We found only a single STS missing with sY139 in 1 out of 40 men with a history of undescended testis. Other STSs that cover the same region were found to be present in this patient. Furthermore, by performing PCR with 50°C annealing temperature (instead of 60°C), we demonstrated the presence of a sY139 product of the expected size, which indicates that the missing signal was probably due to a sequence variation in the primer binding site.
Previously, a single missing STS has been reported to occur in 1% of fertile men. However, it is not easy to judge whether these single missing STSs represent rare sequence variants, more frequent polymorphisms, or real deletions. Although the nature of each single missing STS found in the literature has not been revealed, they are most likely to be considered as polymorphisms. The prevalence of polymorphisms may vary between the different populations. This assumption has been supported by a very recent study, which found a single STS missing for sY84 in Asian fertile men (1/117) (12), even though this STS previously was suggested not to be polymorphic (5). Thus, the pathogenetic significance of these single missing STSs remains to be determined. Bianchi et al. have hypothesized that fertile men having Y microdeletions/single STS missing could be carriers of deletion-mosaicisms with susceptibility to testicular cancer development (8).
Since no contiguous, real Y microdeletions were found in the study population, it seems less likely that deletions on the Y chromosome is an important common etiology to both spermatogenic failure on one side and testicular malignancy or undescent on the other. However, it remains to be determined whether the men having a single missing STS have a potential risk factor for testicular cancer or undescended testis.
ACKNOWLEDGMENTS
The financial support of Novo Nordisk, Danish Medical Research Council, Aarhus Universitet Forskningsinititativ, and Institute of Experimental Clinical Research at Aarhus University are gratefully acknowledged. We thank Anne Ringgaard and Margit Palm Lind for their technical support.
REFERENCES
- 1.Forman D, Moller H. Testicular cancer. Cancer Surv. 1994;19/20:323–341. [PubMed] [Google Scholar]
- 2.Moller H, Skakkebaek NE. Testicular cancer and cryptorchidism in relation to prenatal factors: Case–control studies in Denmark. Cancer Causes Control. 1997;8:904–912. doi: 10.1023/A:1018472530653. [DOI] [PubMed] [Google Scholar]
- 3.Moller H, Skakkebaek NE. Risk of testicular cancer in subfertile men: Case–control study. BMJ. 1999;318:559–562. doi: 10.1136/bmj.318.7183.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leissner J, Filipas D, Wolf HK, Fisch M. The undescended testis: Considerations and impact on fertility. BJU Int. 1999;83:885–891. doi: 10.1046/j.1464-410x.1999.00093.x. [DOI] [PubMed] [Google Scholar]
- 5.Kent-First M, Muallem A, Shultz J, Pryor J, Roberts K, Nolten W, et al. Defining regions of the Y-chromosome responsible for male infertility and identification of a fourth AZF region (AZFd) by Y-chromosome microdeletion detection. Mol Reprod Dev. 1999;53:27–41. doi: 10.1002/(SICI)1098-2795(199905)53:1<27::AID-MRD4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Bor P, Hindkjaer J, Ingerslev HJ, Kolvraa S. Multiplex PCR for screening of microdeletions on the Y chromosome. J Assist Reprod Genet. 2001;18:291–298. doi: 10.1023/A:1016618418319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bor P, Hindkjaer J, Kolvraa S, Ingerslev HJ. A new approach for screening for Y microdeletions: Capillary electrophoresis combined with fluorescent multiplex PCR. J Assist Reprod Genet. 2003;20(1):46–51. doi: 10.1023/A:1021215006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi NO, Richard SM, Peltomaki P, Bianchi MS. Mosaic AZF deletions and susceptibility to testicular tumors. Mutat Res. 2002;503:51–62. doi: 10.1016/s0027-5107(02)00072-6. [DOI] [PubMed] [Google Scholar]
- 9.Frydelund L, Vogt P, Leffers H, Schadwinkel A, Daugaard G, Skakkebaek NE, et al. No AZF deletion in 160 patients with testicular germ cell neoplasia. Mol Hum Rep. 2003;9:517–521. doi: 10.1093/molehr/gag069. [DOI] [PubMed] [Google Scholar]
- 10.Fagerli J, Schneck FX, Lee PA, Bellinger MF, Witchel SF. Absence of microdeletions in the Y chromosome in patients with a history of cryptorchidism and azoospermia or oligospermia. Fertil Steril. 1999;71:697–700. doi: 10.1016/S0015-0282(98)00535-4. [DOI] [PubMed] [Google Scholar]
- 11.Foresta C, Moro E, Garolla A, Onisto M, Ferlin A. Y chromosome microdeletions in cryptorchidism and idiopathic infertility. J Clin Endocrinol Metab. 1999;84:3660–3665. doi: 10.1210/jc.84.10.3660. [DOI] [PubMed] [Google Scholar]
- 12.Thornhill AR, Guenther AJ, Barbarotto GM, Session DR, Damario MA, Dumesic DA, et al. False-positive Y-microdeletion result for a fertile male caused by an alteration under a PCR primer. Int J Androl. 2002;25:352–357. doi: 10.1046/j.1365-2605.2002.00377.x. [DOI] [PubMed] [Google Scholar]