Abstract
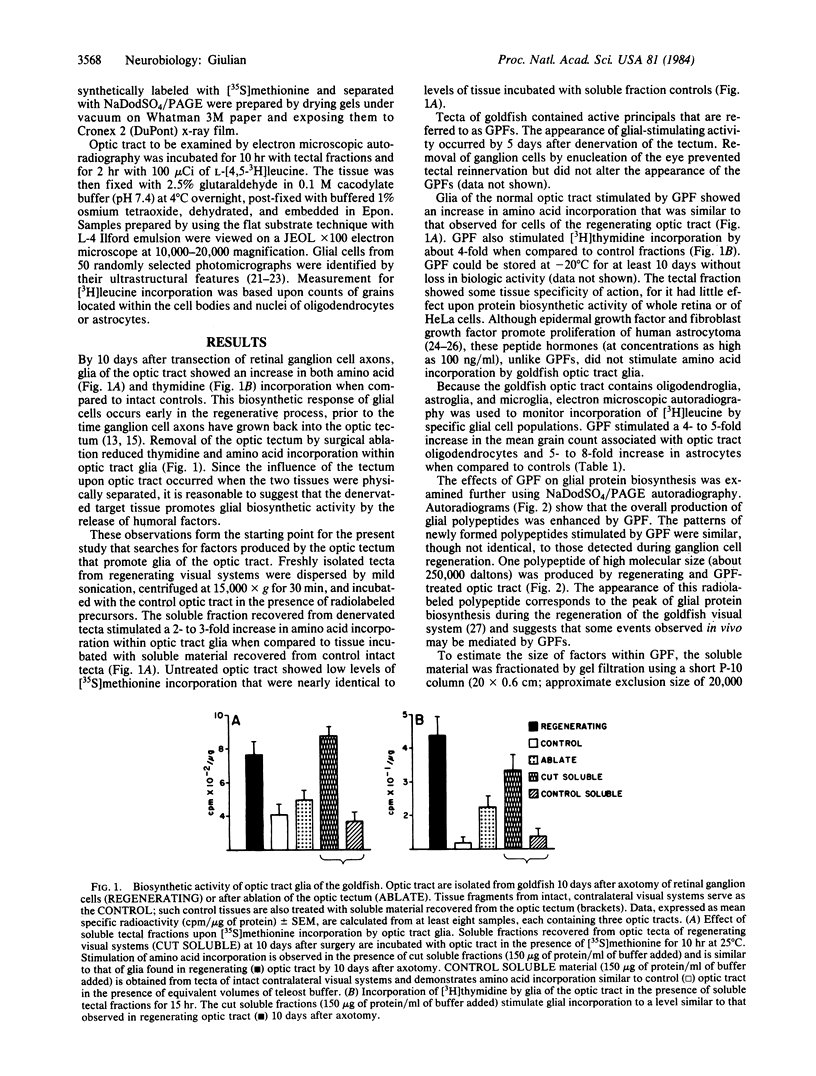
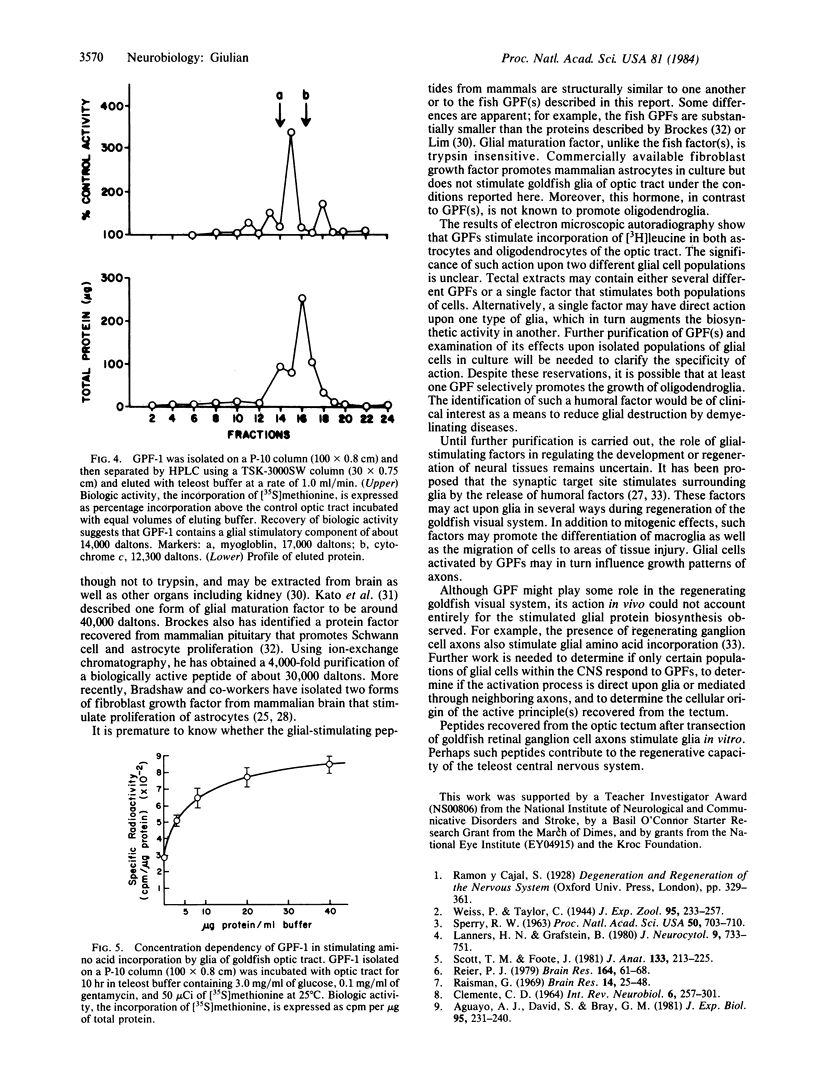
Severed ganglion cell axons of the goldfish retina regrow and form new synaptic connections within their primary target tissue, the optic tectum. During axonal regeneration, optic tract glia show increased incorporation of both thymidine and amino acids. Ablation of the tectum reduces the biosynthetic activity of cells in the optic tract, suggesting that humoral factors released from the tectum may stimulate neighboring glia. A soluble fraction isolated from denervated tecta increases glial incorporation of both thymidine and amino acids by 2- to 3-fold when compared to control cells treated with soluble material from intact tecta. One glial promoting factor, designated GPF -1, is a trypsin-sensitive peptide of about 14,000 daltons. Peptides released from target tissues may help to regulate growth of glial cells during neuronal regeneration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATTARDI D. G., SPERRY R. W. Preferential selection of central pathways by regenerating optic fibers. Exp Neurol. 1963 Jan;7:46–64. doi: 10.1016/0014-4886(63)90093-1. [DOI] [PubMed] [Google Scholar]
- Aguayo A. J., David S., Bray G. M. Influences of the glial environment on the elongation of axons after injury: transplantation studies in adult rodents. J Exp Biol. 1981 Dec;95:231–240. doi: 10.1242/jeb.95.1.231. [DOI] [PubMed] [Google Scholar]
- Benfey M., Aguayo A. J. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature. 1982 Mar 11;296(5853):150–152. doi: 10.1038/296150a0. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Lemke G. E., Balzer D. R., Jr Purification and preliminary characterization of a glial growth factor from the bovine pituitary. J Biol Chem. 1980 Sep 25;255(18):8374–8377. [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- CLEMENTE C. D. REGENERATION IN THE VERTEBRATE CENTRAL NERVOUS SYSTEM. Int Rev Neurobiol. 1964;6:257–301. doi: 10.1016/s0074-7742(08)60771-0. [DOI] [PubMed] [Google Scholar]
- Giulian D., Des Ruisseaux H., Cowburn D. A study of proteins from ganglion cells of the goldfish retina. J Biol Chem. 1980 Jul 10;255(13):6486–6493. [PubMed] [Google Scholar]
- Giulian D., Des Ruisseux H., Cowburn D. Biosynthesis and intra-axonal transport of proteins during neuronal regeneration. J Biol Chem. 1980 Jul 10;255(13):6494–6501. [PubMed] [Google Scholar]
- Giulian D. Isolation of ganglion cells from the retina. Brain Res. 1980 May 5;189(1):135–155. doi: 10.1016/0006-8993(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Growth factors in mammalian cell culture. Annu Rev Biochem. 1976;45:531–558. doi: 10.1146/annurev.bi.45.070176.002531. [DOI] [PubMed] [Google Scholar]
- Kato T., Chiu T. C., Lim R., Troy S. S., Turriff D. E. Multiple molecular forms of glia maturation factor. Biochim Biophys Acta. 1979 Jul 25;579(1):216–227. doi: 10.1016/0005-2795(79)90100-4. [DOI] [PubMed] [Google Scholar]
- Lanners H. N., Grafstein B. Early stages of axonal regeneration in the goldfish optic tract: an electron microscopic study. J Neurocytol. 1980 Dec;9(6):733–751. doi: 10.1007/BF01205016. [DOI] [PubMed] [Google Scholar]
- Lemmon S. K., Riley M. C., Thomas K. A., Hoover G. A., Maciag T., Bradshaw R. A. Bovine fibroblast growth factor: comparison of brain and pituitary preparations. J Cell Biol. 1982 Oct;95(1):162–169. doi: 10.1083/jcb.95.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R. Glia maturation factor. Curr Top Dev Biol. 1980;16:305–322. [PubMed] [Google Scholar]
- Lim R., Mitsunobu K., Li W. K. Maturation-stimulating effect of brain extract and dibutyryl cyclic AMP on dissociated embryonic brain cells in culture. Exp Eye Res. 1973 Apr;79(1):243–246. [PubMed] [Google Scholar]
- Mori S., Leblond C. P. Electron microscopic identification of three classes of oligodendrocytes and a preliminary study of their proliferative activity in the corpus callosum of young rats. J Comp Neurol. 1970 May;139(1):1–28. doi: 10.1002/cne.901390102. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Raisman G. Neuronal plasticity in the septal nuclei of the adult rat. Brain Res. 1969 Jun;14(1):25–48. doi: 10.1016/0006-8993(69)90029-8. [DOI] [PubMed] [Google Scholar]
- Reier P. J. Penetration of grafted astrocytic scars by regenerating optic nerve axons in Xenopus tadpoles. Brain Res. 1979 Mar 23;164:61–68. doi: 10.1016/0006-8993(79)90006-4. [DOI] [PubMed] [Google Scholar]
- SPERRY R. W. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963 Oct;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T. M., Foote J. A study of degeneration, scar formation and regeneration after section of the optic nerve in the frog, Rana pipiens. J Anat. 1981 Sep;133(Pt 2):213–225. [PMC free article] [PubMed] [Google Scholar]
- Singer M., Nordlander R. H., Egar M. Axonal guidance during embryogenesis and regeneration in the spinal cord of the newt: the blueprint hypothesis of neuronal pathway patterning. J Comp Neurol. 1979 May 1;185(1):1–21. doi: 10.1002/cne.901850102. [DOI] [PubMed] [Google Scholar]
- Varon S., Adler R. Nerve growth factors and control of nerve growth. Curr Top Dev Biol. 1980;16:207–252. doi: 10.1016/s0070-2153(08)60157-x. [DOI] [PubMed] [Google Scholar]
- Vaughn J. E., Hinds P. L., Skoff R. P. Electron microscopic studies of Wallerian degeneration in rat optic nerves. I. The multipotential glia. J Comp Neurol. 1970 Oct;140(2):175–206. doi: 10.1002/cne.901400204. [DOI] [PubMed] [Google Scholar]
- WINDLE W. F. Regeneration of axons in the vertebrate central nervous system. Physiol Rev. 1956 Oct;36(4):427–440. doi: 10.1152/physrev.1956.36.4.427. [DOI] [PubMed] [Google Scholar]
- Yoon M. Reorganization of retinotectal projection following surgical operations on the optic tectum in goldfish. Exp Neurol. 1971 Nov;33(2):395–411. doi: 10.1016/0014-4886(71)90031-8. [DOI] [PubMed] [Google Scholar]




