Abstract
The calcium-activated thiol-protease calpain I, which is present in cytosolic and membrane preparations from rat brain, was tested for its capacity to degrade the neuronal spectrin-like protein fodrin. In the presence of micromolar calcium concentrations purified calpain I degraded both purified fodrin and the fodrin present in hippocampal and cerebellar membranes. Fodrin was identified as a high molecular weight protein present in brain membranes by the following criteria: (i) comigration on NaDodSO4/polyacrylamide gels with purified fodrin, (ii) reactivity with antibodies to purified fodrin, and (iii) a proteolytic map following calpain activation comparable to that found after calpain-mediated degradation of purified fodrin. The fodrin breakdown was selective in that calpain I did not affect at least 15 other membrane-associated polypeptides. Fodrin degradation by the protease was rapid and was accompanied by the appearance of a lower molecular weight breakdown product. Calpain I had a high affinity for fodrin, with a Km for degradation of about 50 nM. Purified calpain I also degraded purified spectrin and the spectrin present in erythrocyte membranes. Calpain I-mediated degradation of spectrin-like proteins could provide a mechanism by which brief increases in intracellular free calcium levels modify the structure of the submembraneous cytoskeleton and the distribution of cell surface receptors and alter cell shape.
Full text
PDF
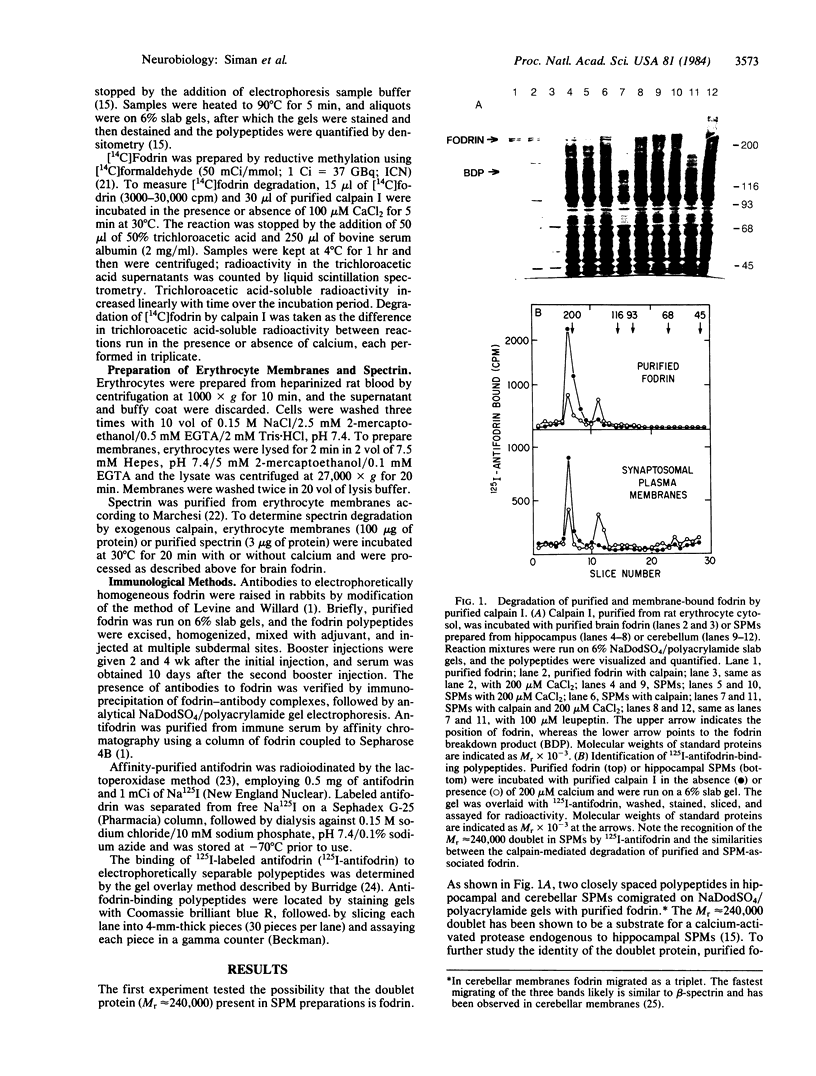
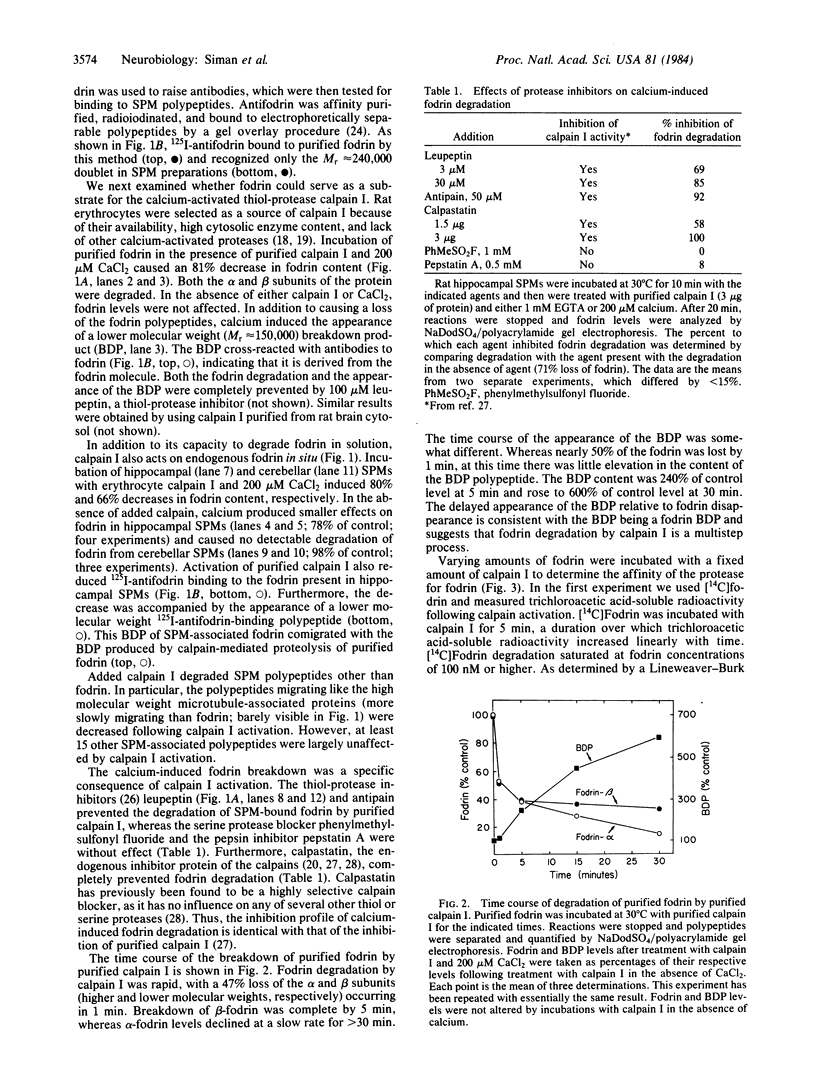
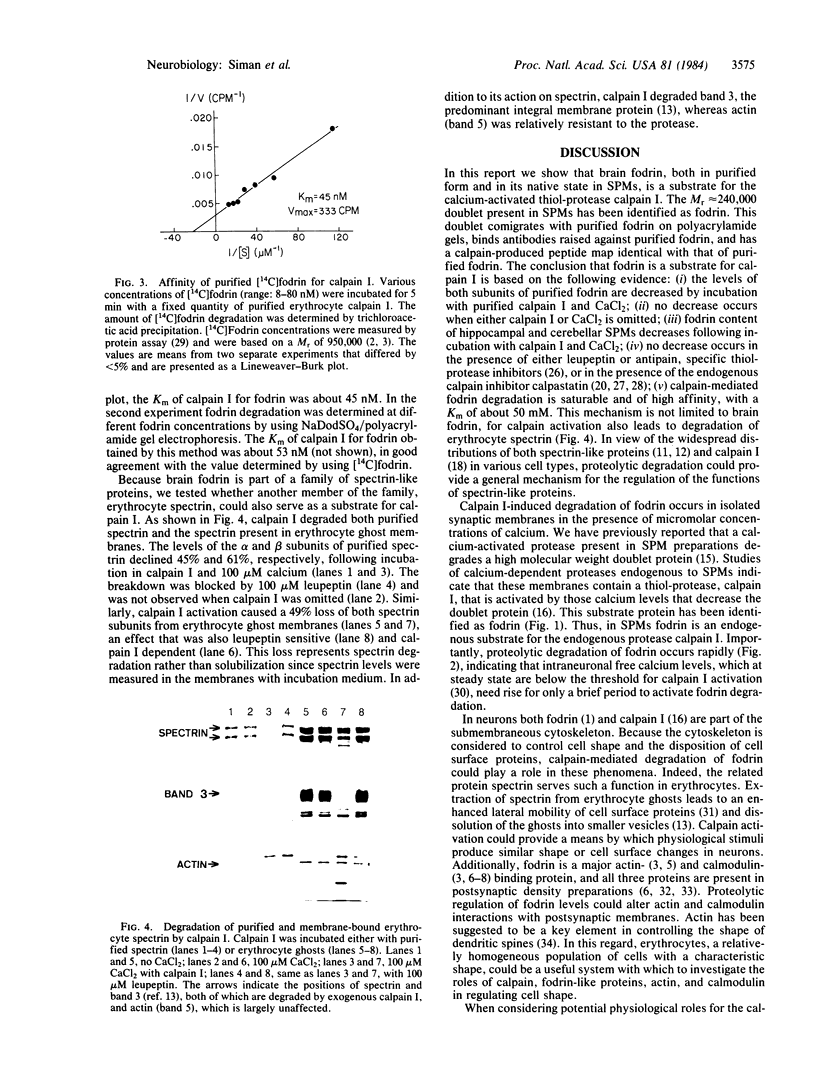

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudry M., Bundman M. C., Smith E. K., Lynch G. S. Micromolar calcium stimulates proteolysis and glutamate binding in rat brain synaptic membranes. Science. 1981 May 22;212(4497):937–938. doi: 10.1126/science.7015504. [DOI] [PubMed] [Google Scholar]
- Baudry M., Kramer K., Fagni L., Recasens M., Lynch G. Classification and properties of acidic amino acid receptors in hippocampus. II. Biochemical studies using a sodium efflux assay. Mol Pharmacol. 1983 Sep;24(2):222–228. [PubMed] [Google Scholar]
- Baudry M., Lynch G. Regulation of hippocampal glutamate receptors: evidence for the involvement of a calcium-activated protease. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2298–2302. doi: 10.1073/pnas.77.4.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Davis J., Fowler W. E. Brain spectrin, a membrane-associated protein related in structure and function to erythrocyte spectrin. Nature. 1982 Sep 9;299(5879):126–131. doi: 10.1038/299126a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Brenner S. L., Korn E. D. Spectrin-actin interaction. Phosphorylated and dephosphorylated spectrin tetramer cross-link F-actin. J Biol Chem. 1979 Sep 10;254(17):8620–8627. [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Burridge K., Kelly T., Mangeat P. Nonerythrocyte spectrins: actin-membrane attachment proteins occurring in many cell types. J Cell Biol. 1982 Nov;95(2 Pt 1):478–486. doi: 10.1083/jcb.95.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin R. K., Bartelt D. C., Siekevitz P. Identification of fodrin as a major calmodulin-binding protein in postsynaptic density preparations. J Cell Biol. 1983 Feb;96(2):443–448. doi: 10.1083/jcb.96.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Siekevitz P. The binding of radio-iodinated calmodulin to proteins on denaturing gels. Ann N Y Acad Sci. 1980;356:73–74. doi: 10.1111/j.1749-6632.1980.tb29600.x. [DOI] [PubMed] [Google Scholar]
- Dipolo R., Requena J., Brinley F. J., Jr, Mullins L. J., Scarpa A., Tiffert T. Ionized calcium concentrations in squid axons. J Gen Physiol. 1976 Apr;67(4):433–467. doi: 10.1085/jgp.67.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Osborn M., Weber K. An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell. 1982 Apr;28(4):843–854. doi: 10.1016/0092-8674(82)90063-0. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Weber K. Erythroid spectrin, brain fodrin, and intestinal brush border proteins (TW-260/240) are related molecules containing a common calmodulin-binding subunit bound to a variant cell type-specific subunit. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4002–4005. doi: 10.1073/pnas.79.13.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. R., Zagon I. S., Kulikowski R. R. Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7570–7574. doi: 10.1073/pnas.78.12.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grab D. J., Berzins K., Cohen R. S., Siekevitz P. Presence of calmodulin in postsynaptic densities isolated from canine cerebral cortex. J Biol Chem. 1979 Sep 10;254(17):8690–8696. [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Fujita M. Purification of a 240 000 Mr calmodulin-binding protein from a microsomal fraction of brain. FEBS Lett. 1981 Sep 14;132(1):144–148. doi: 10.1016/0014-5793(81)80449-8. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Synaptic proteins. Characterization of tubulin and actin and identification of a distinct postsynaptic density polypeptide. J Cell Biol. 1978 Oct;79(1):173–183. doi: 10.1083/jcb.79.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Nelson W. J. Erythrocyte and brain forms of spectrin in cerebellum: distinct membrane-cytoskeletal domains in neurons. Science. 1983 Jun 17;220(4603):1295–1296. doi: 10.1126/science.6190228. [DOI] [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J., Willard M. Redistribution of fodrin (a component of the cortical cytoplasm) accompanying capping of cell surface molecules. Proc Natl Acad Sci U S A. 1983 Jan;80(1):191–195. doi: 10.1073/pnas.80.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. Isolation of spectrin from erythrocyte membranes. Methods Enzymol. 1974;32:275–277. doi: 10.1016/0076-6879(74)32028-9. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L. Canine cardiac calcium-dependent proteases: Resolution of two forms with different requirements for calcium. FEBS Lett. 1980 Jan 1;109(1):129–133. doi: 10.1016/0014-5793(80)81326-3. [DOI] [PubMed] [Google Scholar]
- Melloni E., Sparatore B., Salamino F., Michetti M., Pontremoli S. Cytosolic calcium dependent proteinase of human erythrocytes: formation of an enzyme-natural inhibitor complex induced by Ca2+ ions. Biochem Biophys Res Commun. 1982 Jun 15;106(3):731–740. doi: 10.1016/0006-291x(82)91772-7. [DOI] [PubMed] [Google Scholar]
- Murachi T., Tanaka K., Hatanaka M., Murakami T. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv Enzyme Regul. 1980;19:407–424. doi: 10.1016/0065-2571(81)90026-1. [DOI] [PubMed] [Google Scholar]
- Murakami T., Hatanaka M., Murachi T. The cytosol of human erythrocytes contains a highly Ca2+-sensitive thiol protease (calpain I) and its specific inhibitor protein (calpastatin). J Biochem. 1981 Dec;90(6):1809–1816. doi: 10.1093/oxfordjournals.jbchem.a133659. [DOI] [PubMed] [Google Scholar]
- Palfrey H. C., Schiebler W., Greengard P. A major calmodulin-binding protein common to various vertebrate tissues. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3780–3784. doi: 10.1073/pnas.79.12.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repasky E. A., Granger B. L., Lazarides E. Widespread occurrence of avian spectrin in nonerythroid cells. Cell. 1982 Jul;29(3):821–833. doi: 10.1016/0092-8674(82)90444-5. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Siman R., Baudry M., Lynch G. Purification from synaptosomal plasma membranes of calpain I, a thiol protease activated by micromolar calcium concentrations. J Neurochem. 1983 Oct;41(4):950–956. doi: 10.1111/j.1471-4159.1983.tb09039.x. [DOI] [PubMed] [Google Scholar]
- Sobue K., Kanda K., Inui M., Morimoto K., Kakiuchi S. Actin polymerization induced by calspectin, a calmodulin-binding spectrin-like protein. FEBS Lett. 1982 Nov 8;148(2):221–225. doi: 10.1016/0014-5793(82)80811-9. [DOI] [PubMed] [Google Scholar]
- Takahashi-Nakamura M., Tsuji S., Suzuki K., Imahori K. Purification and characterization of an inhibitor of calcium-activated neutral protease from rabbit skeletal muscle. J Biochem. 1981 Dec;90(6):1583–1589. doi: 10.1093/oxfordjournals.jbchem.a133632. [DOI] [PubMed] [Google Scholar]
- Takano E., Murachi T. Purification and some properties of human erythrocyte calpastatin. J Biochem. 1982 Dec;92(6):2021–2028. doi: 10.1093/oxfordjournals.jbchem.a134134. [DOI] [PubMed] [Google Scholar]
- Toyo-Oka T., Shimizu T., Masaki T. Inhibition of proteolytic activity of calcium activated neutral protease by leupeptin and antipain. Biochem Biophys Res Commun. 1978 May 30;82(2):484–491. doi: 10.1016/0006-291x(78)90900-2. [DOI] [PubMed] [Google Scholar]