Abstract
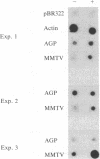
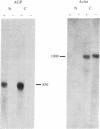
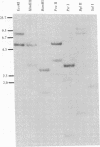
We have studied the glucocorticoid-mediated accumulation of alpha 1-acid glycoprotein (AGP) in mRNA in HTC rat hepatoma cells. In contrast to the well-characterized primary response of mouse mammary tumor virus, in vitro transcription assays in isolated nuclei show that the rate of transcription of the AGP gene is high even in the absence of hormone. Despite the constitutive transcription of the AGP gene, no detectable AGP RNA can be found in either the cytoplasm or the nuclei of untreated cells. Previous experiments have shown that the glucocorticoid induction of AGP RNA requires ongoing protein synthesis. In conjunction with the present study, our data suggest that glucocorticoids stimulate accumulation of AGP RNA by inducing an RNA processing factor that allows production of stable transcripts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Chihara C., Meltzer P., Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:655–662. doi: 10.1101/sqb.1974.038.01.070. [DOI] [PubMed] [Google Scholar]
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Capetanaki Y. G., Ngai J., Flytzanis C. N., Lazarides E. Tissue-specific expression of two mRNA species transcribed from a single vimentin gene. Cell. 1983 Dec;35(2 Pt 1):411–420. doi: 10.1016/0092-8674(83)90174-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Compton J. G., Schrader W. T., O'Malley B. W. Selective binding of chicken progesterone receptor A subunit to a DNA fragment containing ovalbumin gene sequences. Biochem Biophys Res Commun. 1982 Mar 15;105(1):96–104. doi: 10.1016/s0006-291x(82)80015-6. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Feinberg R. F., Sun L. H., Ordahl C. P., Frankel F. R. Identification of glucocorticoid-induced genes in rat hepatoma cells by isolation of cloned cDNA sequences. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5042–5046. doi: 10.1073/pnas.80.16.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone G. L., Payvar F., Yamamoto K. R. Glucocorticoid regulation of protein processing and compartmentalization. Nature. 1982 Nov 18;300(5889):221–225. doi: 10.1038/300221a0. [DOI] [PubMed] [Google Scholar]
- Gorski J., Toft D., Shyamala G., Smith D., Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- Grieninger G., Hertzberg K. M., Pindyck J. Fibrinogen synthesis in serum-free hepatocyte cultures: stimulation by glucocorticoids. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5506–5510. doi: 10.1073/pnas.75.11.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Kawashima T., Stumpf W. E., Jungblut P. W., DeSombre E. R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Mulvihill E. R., LePennec J. P., Chambon P. Chicken oviduct progesterone receptor: location of specific regions of high-affinity binding in cloned DNA fragments of hormone-responsive genes. Cell. 1982 Mar;28(3):621–632. doi: 10.1016/0092-8674(82)90217-3. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Carey N. H. Rapid inactivation of ovalbumin messenger ribonucleic acid after acute withdrawal of estrogen. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2357–2361. doi: 10.1073/pnas.71.6.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca G. A., Taylor J. M. Nucleotide sequence of rat alpha 1-acid glycoprotein messenger RNA. J Biol Chem. 1981 Nov 10;256(21):11199–11202. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Cardiff R. D., Varmus H. E., Yamamoto K. R. Infection of cultured rat hepatoma cells by mouse mammary tumor virus. Cell. 1977 Jan;10(1):11–18. doi: 10.1016/0092-8674(77)90134-9. [DOI] [PubMed] [Google Scholar]
- Ringold G. M. Regulation of mouse mammary tumor virus gene expression by glucocorticoid hormones. Curr Top Microbiol Immunol. 1983;106:79–103. doi: 10.1007/978-3-642-69357-1_4. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Young H. A., Parks W. P. Biochemical and physiological mechanisms in glucocorticoid hormone induction of mouse mammary tumor virus. Virology. 1976 Jan;69(1):148–156. doi: 10.1016/0042-6822(76)90202-6. [DOI] [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe J. D., Vogel S. N., Ryan J. L., McAdam K. P., Rosenstreich D. L. Detection of a mediator derived from endotoxin-stimulated macrohpages that induces the acute phase serum amyloid A response in mice. J Exp Med. 1979 Sep 19;150(3):597–606. doi: 10.1084/jem.150.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. M., Reeve A. E., Huang R. C. Analysis of RNA initiated in isolated mouse myeloma nuclei using purine nucleoside 5'[gamma-S]triphosphates as affinity probes. Cell. 1978 Oct;15(2):615–626. doi: 10.1016/0092-8674(78)90030-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stallcup M. R., Washington L. D. Region-specific initiation of mouse mammary tumor virus RNA synthesis by endogenous RNA polymerase II in preparations of cell nuclei. J Biol Chem. 1983 Mar 10;258(5):2802–2807. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannice J. L., Grove J. R., Ringold G. M. Analysis of glucocorticoid-inducible genes in wild-type and variant rat hepatoma cells. Mol Pharmacol. 1983 May;23(3):779–785. [PubMed] [Google Scholar]
- Vannice J. L., Ringold G. M., McLean J. W., Taylor J. M. Induction of the acute-phase reactant, alpha 1-acid glycoprotein, by glucocorticoids in rat hepatoma cells. DNA. 1983;2(3):205–212. doi: 10.1089/dna.1983.2.205. [DOI] [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R. F., Gordon J. I., Deeley R. G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. A., Shih T. Y., Scolnick E. M., Parks W. P. Steroid induction of mouse mammary tumor virus: effect upon synthesis and degradation of viral RNA. J Virol. 1977 Jan;21(1):139–146. doi: 10.1128/jvi.21.1.139-146.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]