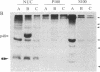
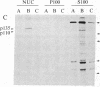
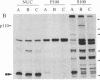
Abstract
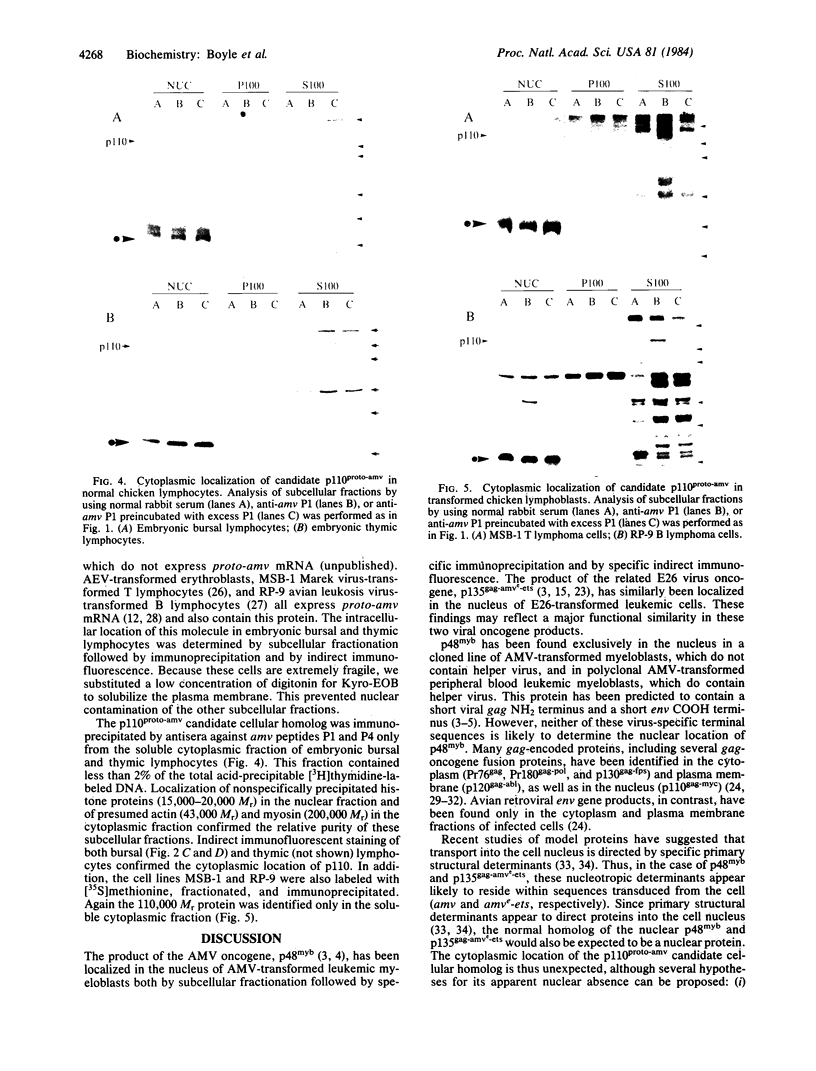
The defective acute leukemia viruses avian myeloblastosis virus (AMV) and E26 virus each contain an inserted cellular sequence related to the same highly conserved cellular gene, proto-amv. The oncogenes of these two retroviruses differ from this cellular proto-oncogene in gene structure, transcript structure, and gene product. The product of the AMV oncogene (myb) is a 48,000 Mr protein, p48myb, encoded by a transduced segment (amv) of proto-amv flanked by short helper-virus-derived terminal sequences. The E26 virus oncogene product is a 135,000 Mr protein, p135gag-amve-ets, encoded by significant portions of a viral structural gene (gag), sequences related to proto-amv (amve), and additional E26-specific sequences (ets) transduced from cellular proto-ets. Both p48myb and p135gag-amve-ets transforming proteins are located in the nucleus of cells transformed by these viruses. A protein of 110,000 Mr which is specifically immunoprecipitated by antisera to amv peptides and may be the product of the normal cellular gene (proto-amv) has been located in the cytoplasm of cells that express proto-amv mRNA.
Full text
PDF

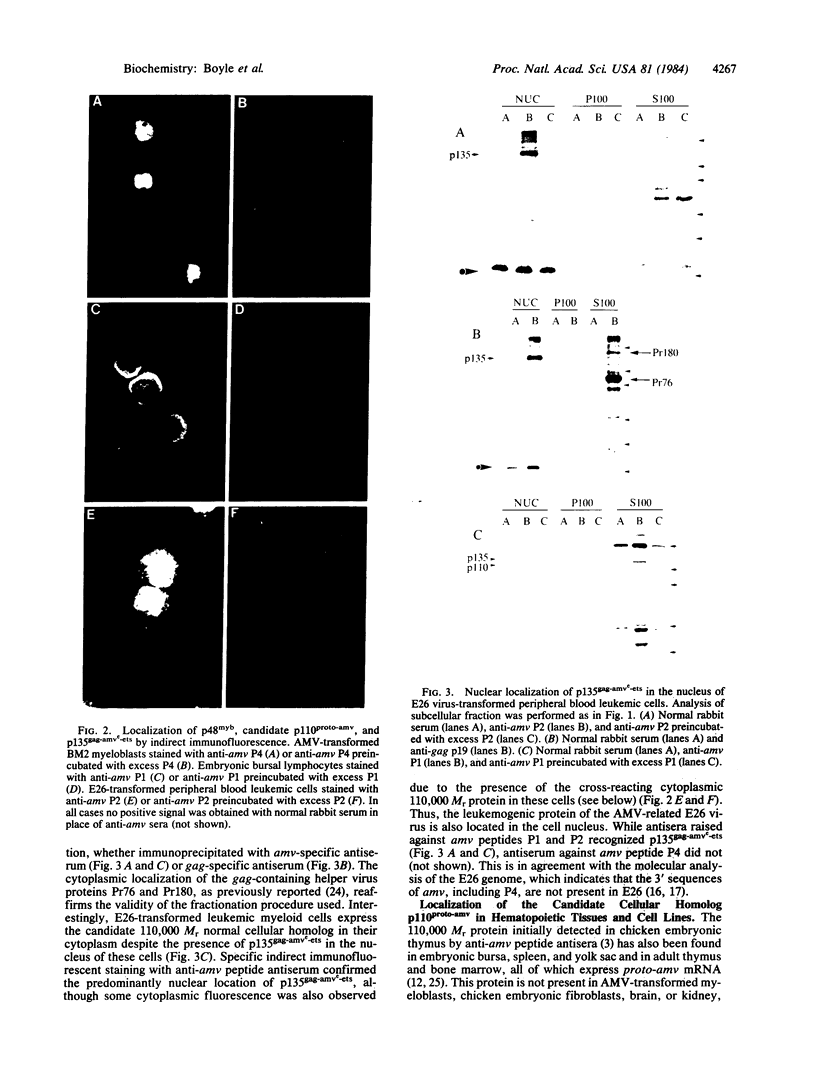

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams H. D., Rohrschneider L. R., Eisenman R. N. Nuclear location of the putative transforming protein of avian myelocytomatosis virus. Cell. 1982 Jun;29(2):427–439. doi: 10.1016/0092-8674(82)90159-3. [DOI] [PubMed] [Google Scholar]
- Akiyama Y., Kato S. Two cell lines from lymphomas of Marek's disease. Biken J. 1974 Sep;17(3):105–116. [PubMed] [Google Scholar]
- Anderson S. J., Furth M., Wolff L., Ruscetti S. K., Sherr C. J. Monoclonal antibodies to the transformation-specific glycoprotein encoded by the feline retroviral oncogene v-fms. J Virol. 1982 Nov;44(2):696–702. doi: 10.1128/jvi.44.2.696-702.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Perbal B., Rushlow K. E., Papas T. S. Avian myeloblastosis virus: a model for the generation of viral oncogenes from potentially oncogenic cellular genetic elements. Folia Biol (Praha) 1983;29(1):18–34. [PubMed] [Google Scholar]
- Beug H., Hayman M. J., Graf T. Myeloblasts transformed by the avian acute leukemia virus E26 are hormone-dependent for growth and for the expression of a putative myb-containing protein, p135 E26. EMBO J. 1982;1(9):1069–1073. doi: 10.1002/j.1460-2075.1982.tb01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birckbichler P. J., Pryme I. F. Fractionation of membrane-bound polysomes, free polysomes, and nuclei from tissue-cultured cells. Eur J Biochem. 1973 Mar 1;33(2):368–373. doi: 10.1111/j.1432-1033.1973.tb02692.x. [DOI] [PubMed] [Google Scholar]
- Bister K., Nunn M., Moscovici C., Perbal B., Baluda M., Duesberg P. H. Acute leukemia viruses E26 and avian myeloblastosis virus have related transformation-specific RNA sequences but different genetic structures, gene products, and oncogenic properties. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3677–3681. doi: 10.1073/pnas.79.12.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Lipsick J. S., Reddy E. P., Baluda M. A. Identification of the leukemogenic protein of avian myeloblastosis virus and of its normal cellular homologue. Proc Natl Acad Sci U S A. 1983 May;80(10):2834–2838. doi: 10.1073/pnas.80.10.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr J. G., Dreyfuss G., Penman S., Buchanan J. M. Association of the src gene product of Rous sarcoma virus with cytoskeletal structures of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3484–3488. doi: 10.1073/pnas.77.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H. Expression of endogenous avian myeloblastosis virus information in different chicken cells. J Virol. 1980 Oct;36(1):162–170. doi: 10.1128/jvi.36.1.162-170.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Hayward W. S., Moscovici C. Size and genetic content of virus-specific RNA in myeloblasts transformed by avian myeloblastosis virus (AMV). Virology. 1981 Apr 15;110(1):128–136. doi: 10.1016/0042-6822(81)90014-3. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M. Nucleocytoplasmic segregation of proteins and RNAs. Cell. 1983 Apr;32(4):1021–1025. doi: 10.1016/0092-8674(83)90285-4. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Donner P., Greiser-Wilke I., Moelling K. Nuclear localization and DNA binding of the transforming gene product of avian myelocytomatosis virus. Nature. 1982 Mar 18;296(5854):262–269. doi: 10.1038/296262a0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Moscovici C. Genetic structure of avian myeloblastosis virus, released from transformed myeloblasts as a defective virus particle. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5120–5124. doi: 10.1073/pnas.77.9.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. A., Wang E., Hanafusa H. Cytoplasmic localization of the transforming protein of Fujinami sarcoma virus: salt-sensitive association with subcellular components. J Virol. 1983 Feb;45(2):782–791. doi: 10.1128/jvi.45.2.782-791.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Bishop J. M. Structure and transcription of the cellular homolog (c-myb) of the avian myeloblastosis virus transforming gene (v-myb). J Virol. 1983 Apr;46(1):212–220. doi: 10.1128/jvi.46.1.212-220.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Fanshier L., Bishop J. M., Moscovici C., Moscovici M. G. The genome and the intracellular RNAs of avian myeloblastosis virus. Cell. 1981 Jan;23(1):279–290. doi: 10.1016/0092-8674(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Ramsay G. M., Savin K., Kitchener G., Graf T., Beug H. Identification and characterization of the avian erythroblastosis virus erbB gene product as a membrane glycoprotein. Cell. 1983 Feb;32(2):579–588. doi: 10.1016/0092-8674(83)90477-4. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Ramsay G., Bishop J. M., Moscovici M. G., Moscovici C., McGrath J. P., Levinson A. D. The product of the retroviral transforming gene v-myb is a truncated version of the protein encoded by the cellular oncogene c-myb. Cell. 1983 Jun;33(2):345–355. doi: 10.1016/0092-8674(83)90416-6. [DOI] [PubMed] [Google Scholar]
- Krueger J. G., Wang E., Goldberg A. R. Evidence that the src gene product of Rous sarcoma virus is membrane associated. Virology. 1980 Feb;101(1):25–40. doi: 10.1016/0042-6822(80)90480-8. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Sefton B. M., Hunter T., Walter G., Singer S. J. Immunofluorescent localization of the transforming protein of Rous sarcoma virus with antibodies against a synthetic src peptide. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5322–5326. doi: 10.1073/pnas.79.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Nigg E. A., Hunter T. The transforming protein of Moloney murine sarcoma virus is a soluble cytoplasmic protein. Cell. 1983 May;33(1):161–172. doi: 10.1016/0092-8674(83)90345-8. [DOI] [PubMed] [Google Scholar]
- Perbal B., Cline J. M., Hillyard R. L., Baluda M. A. Organization of chicken DNA sequences homologous to the transforming gene of avian myeloblastosis virus. II. Isolation and characterization of lambda proto-amv DNA recombinant clones from a library of leukemic chicken DNA. J Virol. 1983 Mar;45(3):925–940. doi: 10.1128/jvi.45.3.925-940.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Rushlow K. E., Lautenberger J. A., Papas T. S., Baluda M. A., Perbal B., Chirikjian J. G., Reddy E. P. Nucleotide sequence of the transforming gene of avian myeloblastosis virus. Science. 1982 Jun 25;216(4553):1421–1423. doi: 10.1126/science.6283631. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Briskin M. J., Hillyard R. L., Baluda M. A. Identification of the avian myeloblastosis virus genome. II. Restriction endonuclease analysis of DNA from lambda proviral recombinants and leukemic myeoblast clones. J Virol. 1980 Nov;36(2):325–336. doi: 10.1128/jvi.36.2.325-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Strommer J. N., Hillyard R. L., Komaromy M. C., Baluda M. A. Cellular sequences are present in the presumptive avian myeloblastosis virus genome. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5177–5181. doi: 10.1073/pnas.77.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Jay G., Pastan I. Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1979 Sep;18(1):125–134. doi: 10.1016/0092-8674(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Baltimore D. Preparation of syngeneic tumor regressor serum reactive with the unique determinants of the Abelson murine leukemia virus-encoded P120 protein at the cell surface. J Virol. 1979 Sep;31(3):776–784. doi: 10.1128/jvi.31.3.776-784.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]