Abstract
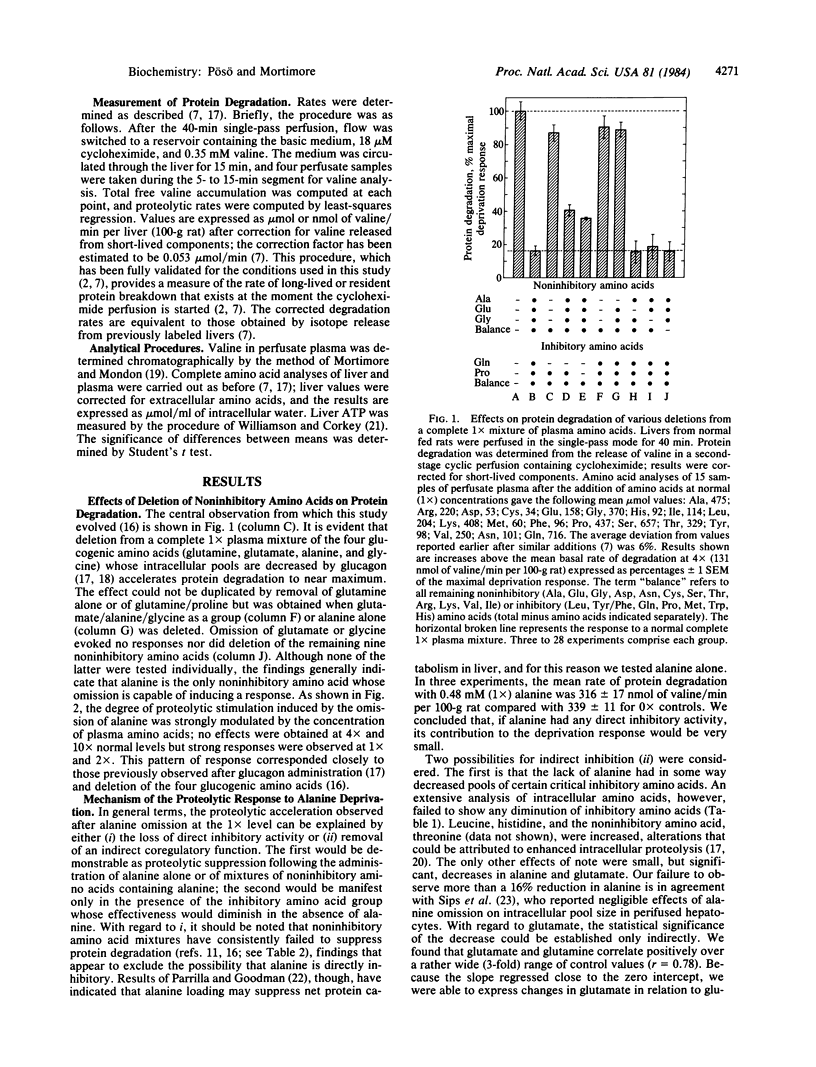
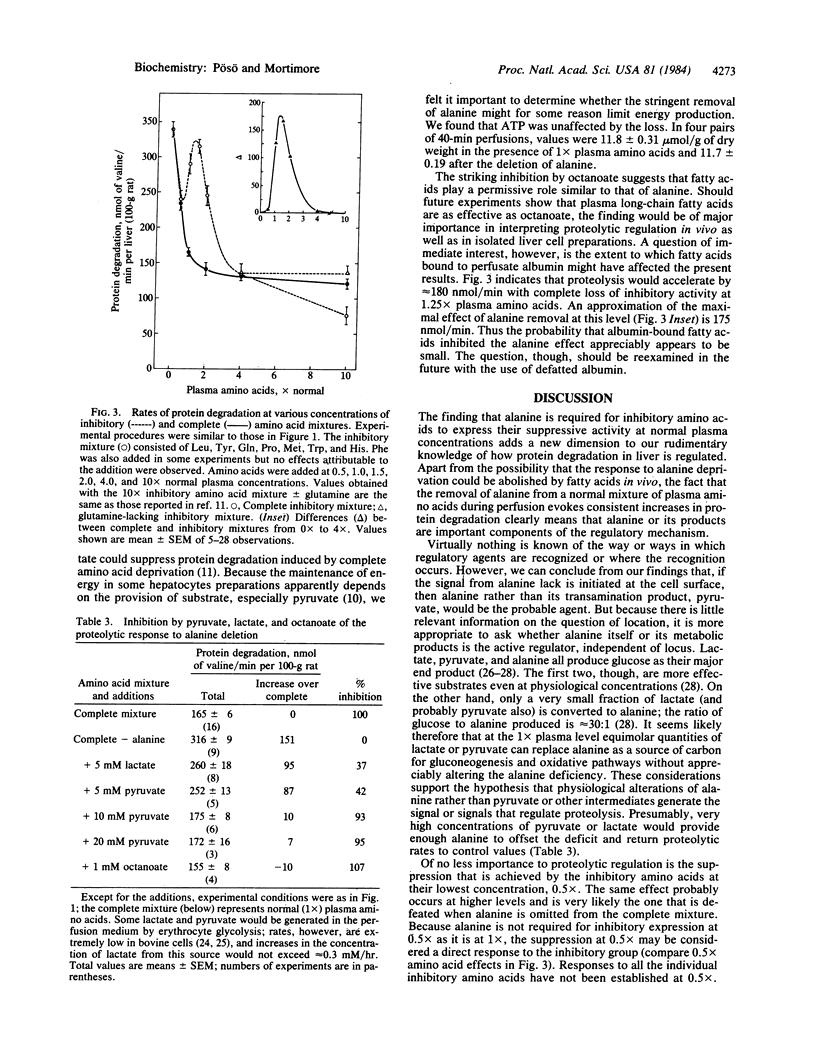
Protein degradation in liver is actively controlled by a small group of inhibitory amino acids--leucine, tyrosine (or phenylalanine), glutamine, proline, histidine, tryptophan, and methionine. Other evidence, however, suggests that one or more of the remaining 12 noninhibitory amino acids is also required for suppression of proteolysis at normal concentrations. This question was investigated in livers of fed rats perfused in the single-pass mode. The deletion of alanine at normal (1x), but not at 4x or 10x normal, plasma amino acid concentrations evoked a near-maximal acceleration of protein degradation. No other noninhibitory amino acid was effective. Because alanine alone was not directly inhibitory and its omission was not associated with a decrease in inhibitory amino acid pools, alanine was presumed to act as a coregulator in the expression of inhibitory activity. When tested alone, the inhibitory group was as effective as the complete mixture at 0.5x and 4x levels, but it lost its suppressive ability within a narrow zone of concentration centered slightly above 1x. The addition of 1x (0.48 mM) alanine completely restored the inhibition. Pyruvate and lactate could be effectively substituted, but only at concentrations 10-20 times greater than that of alanine. These, together with earlier findings, indicate the existence of a regulatory complex that recognizes specific amino acids and transmits positive and negative signals to proteolytic sites. The results also suggest that alanine can provide an important regulatory link between energy demands and protein degradation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Grinde B., Seglen P. O. Differential effects of proteinase inhibitors and amines on the lysosomal and non-lysosomal pathways of protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1980 Sep 17;632(1):73–86. doi: 10.1016/0304-4165(80)90250-0. [DOI] [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Inhibition of protein degradation in isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):399–407. doi: 10.1042/bj1640399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson N. J., Mortimore G. E. Suppression of cytoplasmic protein uptake by lysosomes as the mechanism of protein regain in livers of starved-refed mice. J Biol Chem. 1982 Aug 25;257(16):9548–9554. [PubMed] [Google Scholar]
- Hutson S. M., Harper A. E. Blood and tissue branched-chain amino and alpha-keto acid concentrations: effect of diet, starvation, and disease. Am J Clin Nutr. 1981 Feb;34(2):173–183. doi: 10.1093/ajcn/34.2.173. [DOI] [PubMed] [Google Scholar]
- Kim H. D. Cow red blood cells. III. Postnatal adaptation of energy metabolism in the calf red blood cells. Biochim Biophys Acta. 1979 Nov 15;588(1):44–54. doi: 10.1016/0304-4165(79)90369-6. [DOI] [PubMed] [Google Scholar]
- Mallette L. E., Exton J. H., Park Effects of glucagon on amino acid transport and utilization in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5724–5728. [PubMed] [Google Scholar]
- Martin G., Baverel G. Lactate, alanine and glutamine metabolism in isolated canine pup liver cells. Biochim Biophys Acta. 1983 Oct 18;760(2):230–237. doi: 10.1016/0304-4165(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Mortimore G. E., Hutson N. J., Surmacz C. A. Quantitative correlation between proteolysis and macro- and microautophagy in mouse hepatocytes during starvation and refeeding. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2179–2183. doi: 10.1073/pnas.80.8.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Mortimore G. E., Woodside K. H., Henry J. E. Compartmentation of free valine and its relation to protein turnover in perfused rat liver. J Biol Chem. 1972 May 10;247(9):2776–2784. [PubMed] [Google Scholar]
- Neff N. T., DeMartino G. N., Goldberg A. L. The effect of protease inhibitors and decreased temperature on the degradation of different classes of proteins in cultured hepatocytes. J Cell Physiol. 1979 Dec;101(3):439–457. doi: 10.1002/jcp.1041010311. [DOI] [PubMed] [Google Scholar]
- Parrilla R., Goodman M. N. Nitrogen metabolism in the isolated perfused rat liver. Nitrogen balance, redox state and rates of proteolysis. Biochem J. 1974 Mar;138(3):341–348. doi: 10.1042/bj1380341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pösö A. R., Schworer C. M., Mortimore G. E. Acceleration of proteolysis in perfused rat liver by deletion of glucogenic amino acids: regulatory role of glutamine. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1433–1439. doi: 10.1016/s0006-291x(82)80159-9. [DOI] [PubMed] [Google Scholar]
- Pösö A. R., Wert J. J., Jr, Mortimore G. E. Multifunctional control of amino acids of deprivation-induced proteolysis in liver. Role of leucine. J Biol Chem. 1982 Oct 25;257(20):12114–12120. [PubMed] [Google Scholar]
- Ross B. D., Hems R., Krebs H. A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967 Mar;102(3):942–951. doi: 10.1042/bj1020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schworer C. M., Mortimore G. E. Glucagon-induced autophagy and proteolysis in rat liver: mediation by selective deprivation of intracellular amino acids. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3169–3173. doi: 10.1073/pnas.76.7.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schworer C. M., Shiffer K. A., Mortimore G. E. Quantitative relationship between autophagy and proteolysis during graded amino acid deprivation in perfused rat liver. J Biol Chem. 1981 Jul 25;256(14):7652–7658. [PubMed] [Google Scholar]
- Scornik O. A. Faster protein degradation in response to decreases steady state levels of amino acylation of tRNAHis in Chinese hamster ovary cells. J Biol Chem. 1983 Jan 25;258(2):882–886. [PubMed] [Google Scholar]
- Scornik O. A., Ledbetter M. L., Malter J. S. Role of aminoacylation of histidyl-tRNA in the regulation of protein degradation in Chinese hamster ovary cells. J Biol Chem. 1980 Jul 10;255(13):6322–6329. [PubMed] [Google Scholar]
- Seglen P. O., Solheim A. E., Grinde B., Gordon P. B., Schwarze P. E., Gjessing R., Poli A. Amino acid control of protein synthesis and degradation in isolated rat hepatocytes. Ann N Y Acad Sci. 1980;349:1–17. doi: 10.1111/j.1749-6632.1980.tb29510.x. [DOI] [PubMed] [Google Scholar]
- Seider M. J., Kim H. D. Cow red blood cells. I. Effect of purines, pyrimidines, and nucleosides in bovine red cell glycolysis. Am J Physiol. 1979 May;236(5):C255–C261. doi: 10.1152/ajpcell.1979.236.5.C255. [DOI] [PubMed] [Google Scholar]
- Shiman R., Gray D. W. Substrate activation of phenylalanine hydroxylase. A kinetic characterization. J Biol Chem. 1980 May 25;255(10):4793–4800. [PubMed] [Google Scholar]
- Shiman R., Mortimore G. E., Schworer C. M., Gray D. W. Regulation of phenylalanine hydroxylase activity by phenylalanine in vivo, in vitro, and in perfused rat liver. J Biol Chem. 1982 Oct 10;257(19):11213–11216. [PubMed] [Google Scholar]
- Sips H. J., Groen A. K., Tager J. M. Plasma-membrane transport of alanine is rate-limiting for its metabolism in rat-liver parenchymal cells. FEBS Lett. 1980 Oct 6;119(2):271–274. doi: 10.1016/0014-5793(80)80269-9. [DOI] [PubMed] [Google Scholar]
- Sommercorn J. M., Swick R. W. Protein degradation in primary monolayer cultures of adult rat hepatocytes. Further evidence for the regulation of protein degradation by amino acids. J Biol Chem. 1981 May 25;256(10):4816–4821. [PubMed] [Google Scholar]
- Ward W. F., Cox J. R., Mortimore G. E. Lysosomal sequestration of intracellular protein as a regulatory step in hepatic proteolysis. J Biol Chem. 1977 Oct 10;252(19):6955–6961. [PubMed] [Google Scholar]
- Woodside K. H., Mortimore G. E. Suppression of protein turnover by amino acids in the perfused rat liver. J Biol Chem. 1972 Oct 25;247(20):6474–6481. [PubMed] [Google Scholar]



