Abstract
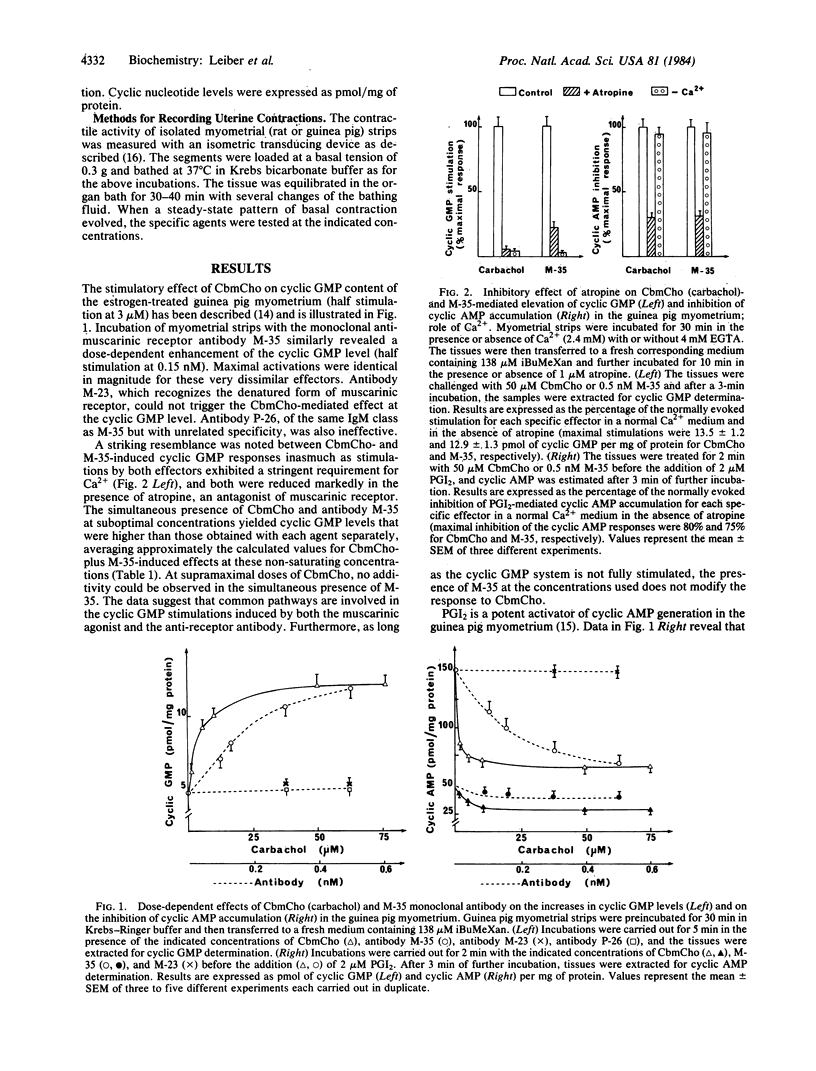
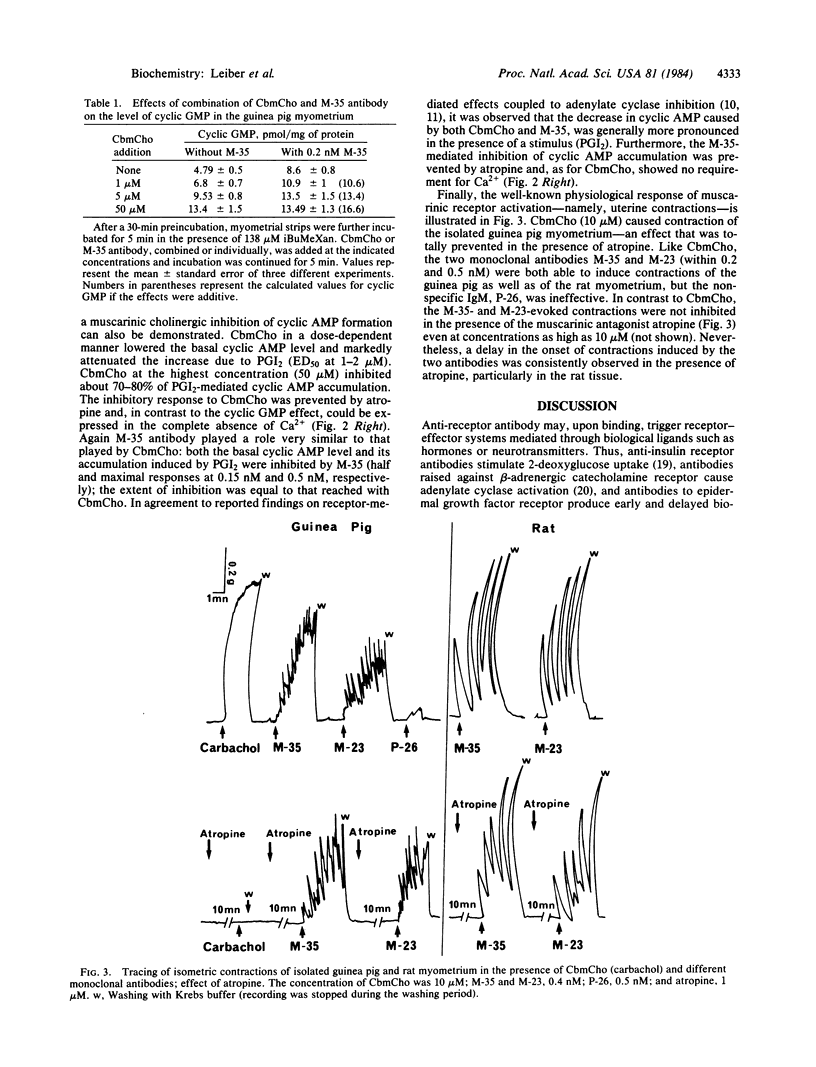
Monoclonal antibody M-35, which immunoprecipitates native calf brain acetylcholine muscarinic receptor, mimics agonist stimulation of the intact guinea pig myometrium: the antibody, just like carbamoylcholine hydrochloride, causes a rise in intracellular cyclic GMP content, an inhibition of cyclic AMP accumulation due to prostacyclin, and induces uterine contractions. Another antibody, M-23, which reacts with the denatured muscarinic receptor, is devoid of agonist-like activity at the cyclic nucleotide level but is still able to induce contractions of both rat and guinea pig myometrium. The cyclic nucleotide changes caused by both carbamoylcholine and antibody M-35 are inhibited by atropine; this antagonist, which blocks carbamoylcholine-mediated contractions, fails however, to prevent contractions induced by antibodies M-35 and M-23. These results suggest that the information necessary to transmit muscarinic signals is entirely contained in the receptor and that ligands only act to trigger the biological response. The data also imply that the muscarinic receptors of the myometrium are coupled to multiple effector systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., De Backer J. P., Guillet J. C., Vanderheyden P., Vauquelin G., Strosberg A. D. Purification of muscarinic acetylcholine receptors by affinity chromatography. EMBO J. 1983;2(4):499–504. doi: 10.1002/j.1460-2075.1983.tb01453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André C., Guillet J. G., De Backer J. P., Vanderheyden P., Hoebeke J., Strosberg A. D. Monoclonal antibodies against the native or denatured forms of muscarinic acetylcholine receptors. EMBO J. 1984 Jan;3(1):17–21. doi: 10.1002/j.1460-2075.1984.tb01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Phosphatidylinositol hydrolysis: a multifunctional transducing mechanism. Mol Cell Endocrinol. 1981 Nov;24(2):115–140. doi: 10.1016/0303-7207(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Birdsall N. J., Berrie C. P., Burgen A. S., Hulme E. C. Modulation of the binding properties of muscarinic receptors: evidence for receptor-effector coupling. Adv Biochem Psychopharmacol. 1980;21:107–116. [PubMed] [Google Scholar]
- Cailla H. L., Vannier C. J., Delaage M. A. Guanosine 3', 5'-cyclicmonophosphate assay at 10(-15)-mole level. Anal Biochem. 1976 Jan;70(1):195–202. doi: 10.1016/s0378-5173(83)90100-x. [DOI] [PubMed] [Google Scholar]
- Changeux J. P. The acetylcholine receptor: an "allosteric" membrane protein. Harvey Lect. 1979 1980;75:85–254. [PubMed] [Google Scholar]
- Couraud P. O., Delavier-Klutchko C., Durieu-Trautmann O., Strosberg A. D. "Antibodies raised against beta-adrenergic receptors stimulate adenylate cyclase". Biochem Biophys Res Commun. 1981 Apr 30;99(4):1295–1302. doi: 10.1016/0006-291x(81)90760-9. [DOI] [PubMed] [Google Scholar]
- Diamond J. Role of cyclic nucleotides in control of smooth muscle contraction. Adv Cyclic Nucleotide Res. 1978;9:327–340. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. Inhibition of adenylate cyclase by hormones and neurotransmitters. Adv Cyclic Nucleotide Res. 1981;14:173–187. [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Flier J. S., Grunfeld C., Harmon J. T., Harrison L. C., Karlsson F. A., Kasuga M., King G. L., Lang U. C. Insulin receptors, receptor antibodies, and the mechanism of insulin action. Recent Prog Horm Res. 1981;37:477–538. doi: 10.1016/b978-0-12-571137-1.50015-3. [DOI] [PubMed] [Google Scholar]
- Laduron P. M., Verwimp M., Leysen J. E. Stereospecific in vitro binding of [3H]dexetimide to brain muscarinic receptors. J Neurochem. 1979 Feb;32(2):421–427. doi: 10.1111/j.1471-4159.1979.tb00366.x. [DOI] [PubMed] [Google Scholar]
- Leiber D., Harbon S. The relationship between the carbachol stimulatory effect on cyclic GMP content and activation by fatty acid hydroperoxides of a soluble guanylate cyclase in the guinea pig myometrium. Mol Pharmacol. 1982 May;21(3):654–663. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Sumikawa K. Properties of acetylcholine receptors translated by cat muscle mRNA in Xenopus oocytes. EMBO J. 1982;1(11):1307–1312. doi: 10.1002/j.1460-2075.1982.tb01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Libermann T. A., Lax I., Yarden Y., Schlessinger J. Biological role of epidermal growth factor-receptor clustering. Investigation with monoclonal anti-receptor antibodies. J Biol Chem. 1983 Jan 25;258(2):846–853. [PubMed] [Google Scholar]
- Vauquelin G., André C., De Backer J. P., Laduron P., Strosberg A. D. Agonist-mediated conformational changes of muscarinic receptors in rat brain. Eur J Biochem. 1982 Jun 15;125(1):117–124. doi: 10.1111/j.1432-1033.1982.tb06658.x. [DOI] [PubMed] [Google Scholar]
- Vesin M. F., Khac L. D., Harbon S. Prostacyclin as an endogenous modulator of adenosine cyclic 3',5'-monophosphate levels in rat myometrium and endometrium. Mol Pharmacol. 1979 Nov;16(3):823–840. [PubMed] [Google Scholar]
- Vesin M. F., Leiber D., Harbon S. Contribution of lipoxygenase and cyclooxygenase metabolites in the modulation of cyclic nucleotides in the guinea pig myometrium. Differential effects of carbachol and ionophore A23187. Prostaglandins. 1982 Dec;24(6):851–871. doi: 10.1016/0090-6980(82)90065-x. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Mayes E. L., Stroobant P., Bennet P. L., Young S., Goodfellow P. N., Banting G. S., Ozanne B. A monoclonal antibody to the human epidermal growth factor receptor. J Cell Biochem. 1982;20(2):149–161. doi: 10.1002/jcb.240200207. [DOI] [PubMed] [Google Scholar]


