Abstract
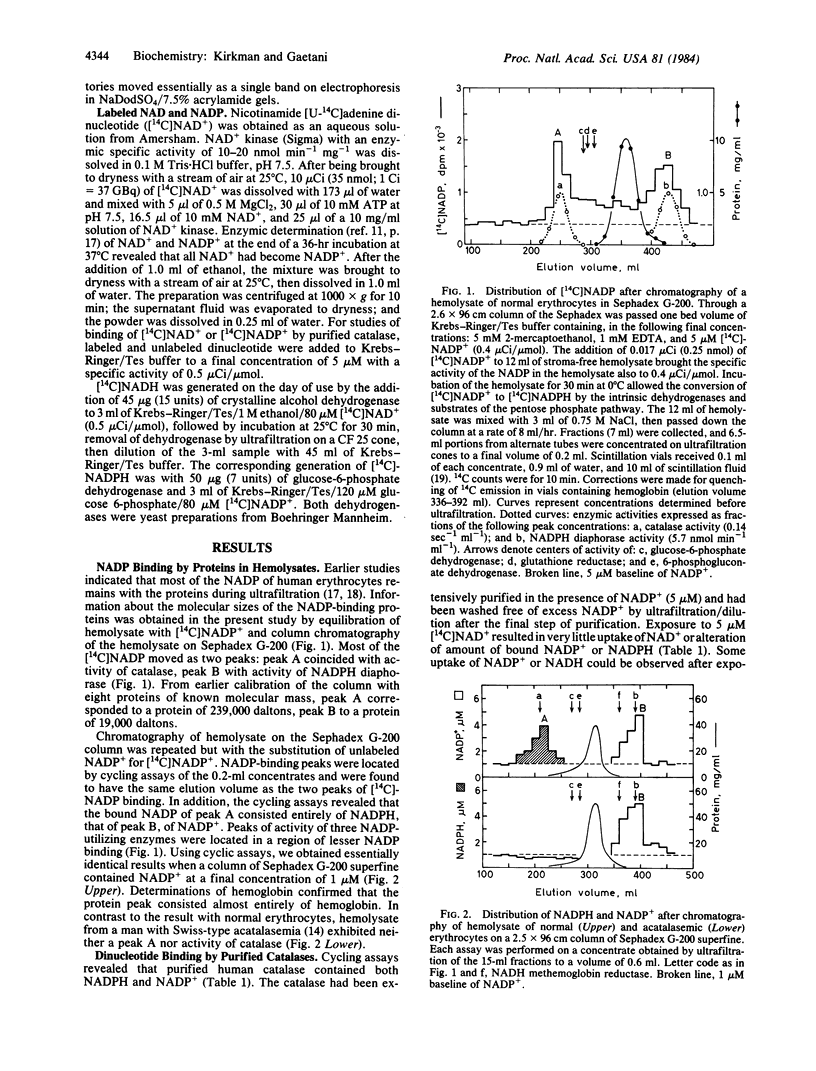
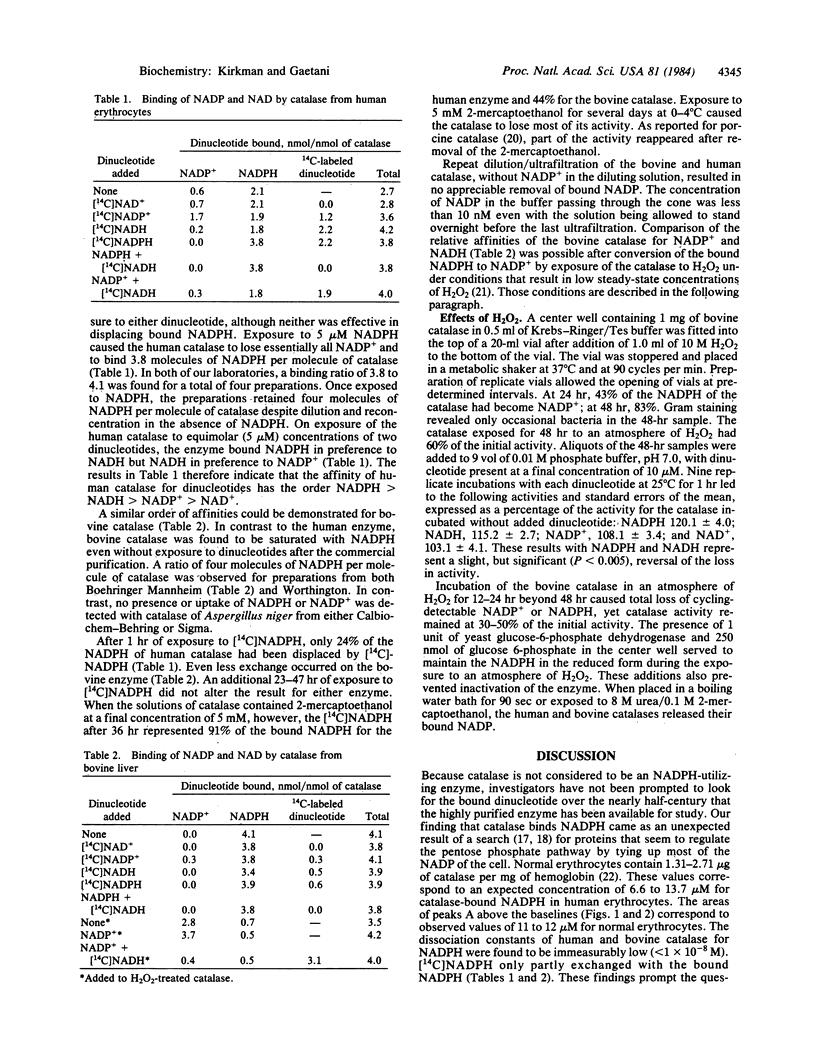
Catalases (H2O2:H2O2 oxidoreductase, EC 1.11.1.6) from many species are known to be tetramers of 60,000-dalton subunits, with four heme groups per tetramer. Previous authors have determined the amino acid sequence and three-dimensional structure of bovine liver catalase. Studies of the regulation of the pentose phosphate pathway led the present authors to a search for proteins that bind NADP+ and NADPH in human erythrocytes. An unexpected result of that search was the finding that a major reservoir of bound NADPH in human erythrocytes is catalase. Each tetrameric molecule of human or bovine catalase contains four molecules of tightly bound NADPH. The binding sites have the relative affinities NADPH greater than NADH greater than NADP+ greater than NAD+. NADPH does not seem to be essential for the enzymic conversion of H2O2 to O2 and water but does provide protection of catalase against inactivation by H2O2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Yoseph Y., Shapira E. Specific immunoassay for quantitative determination of human erythrocyte catalase. J Lab Clin Med. 1973 Jan;81(1):133–139. [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GLUTATHIONE PEROXIDASE: THE PRIMARY AGENT FOR THE ELIMINATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES. Biochemistry. 1963 Nov-Dec;2:1420–1428. doi: 10.1021/bi00906a038. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. Glucose-6-phosphate dehydrogenase and detoxification of hydrogen peroxide in human erythrocytes. Science. 1961 Dec 1;134(3492):1756–1757. doi: 10.1126/science.134.3492.1756. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Boraas M., Etkin N. L. Catalase activity and red cell metabolism. Adv Exp Med Biol. 1972;28:121–131. doi: 10.1007/978-1-4684-3222-0_8. [DOI] [PubMed] [Google Scholar]
- Gaetani G. D., Parker J. C., Kirkman H. N. Intracellular restraint: a new basis for the limitation in response to oxidative stress in human erythrocytes containing low-activity variants of glucose-6-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3584–3587. doi: 10.1073/pnas.71.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetani G. F., Mareni C., Salvidio E., Galiano S., Meloni T., Arese P. Favism: erythrocyte metabolism during haemolysis and reticulocytosis. Br J Haematol. 1979 Sep;43(1):39–48. doi: 10.1111/j.1365-2141.1979.tb03717.x. [DOI] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of catalase. Catalysis of coupled oxidation of alcohols. Biochem J. 1945;39(4):293–301. [PMC free article] [PubMed] [Google Scholar]
- Kikuchi-Torii K., Hayashi S., Nakamoto H., Nakamura S. Properties of Aspergillus niger catalase. J Biochem. 1982 Nov;92(5):1449–1456. doi: 10.1093/oxfordjournals.jbchem.a134069. [DOI] [PubMed] [Google Scholar]
- Kirkman H. N., Wilson W. G., Clemons E. H. Regulation of glucose-6-phosphate dehydrogenase. I. Intact red cells. J Lab Clin Med. 1980 Jun;95(6):877–887. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murthy M. R., Reid T. J., 3rd, Sicignano A., Tanaka N., Rossmann M. G. Structure of beef liver catalase. J Mol Biol. 1981 Oct 25;152(2):465–499. doi: 10.1016/0022-2836(81)90254-0. [DOI] [PubMed] [Google Scholar]
- Mörikofer-Zwez S., Cantz M., Kaufmann H., von Wartburg J. P., Aebi H. Heterogeneity of erythrocyte catalase. Correlations between sulfhydryl group content, chromatographic and electrophoretic properties. Eur J Biochem. 1969 Nov;11(1):49–57. doi: 10.1111/j.1432-1033.1969.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. R., Shelton J. B., Robberson B., Apell G., Fang R. S., Bonaventura J. The complete amino acid sequence of bovine liver catalase and the partial sequence of bovine erythrocyte catalase. Arch Biochem Biophys. 1982 Mar;214(1):397–421. doi: 10.1016/0003-9861(82)90044-3. [DOI] [PubMed] [Google Scholar]
- Sullivan S. G., McMahon S., Stern A. Restoration of red cell catalase activity by glucose metabolism after exposure to a vitamin K analog. Biochem Pharmacol. 1979 Dec 1;28(23):3403–3407. doi: 10.1016/0006-2952(79)90079-0. [DOI] [PubMed] [Google Scholar]
- Takeda A., Miyahara T., Hachimori A., Samejima T. The interactions of thiol compounds with porcine erythrocyte catalase. J Biochem. 1980 Feb;87(2):429–439. doi: 10.1093/oxfordjournals.jbchem.a132763. [DOI] [PubMed] [Google Scholar]
- Vainshtein B. K., Melik-Adamyan W. R., Barynin V. V., Vagin A. A., Grebenko A. I. Three-dimensional structure of the enzyme catalase. Nature. 1981 Oct 1;293(5831):411–412. doi: 10.1038/293411a0. [DOI] [PubMed] [Google Scholar]



