Abstract
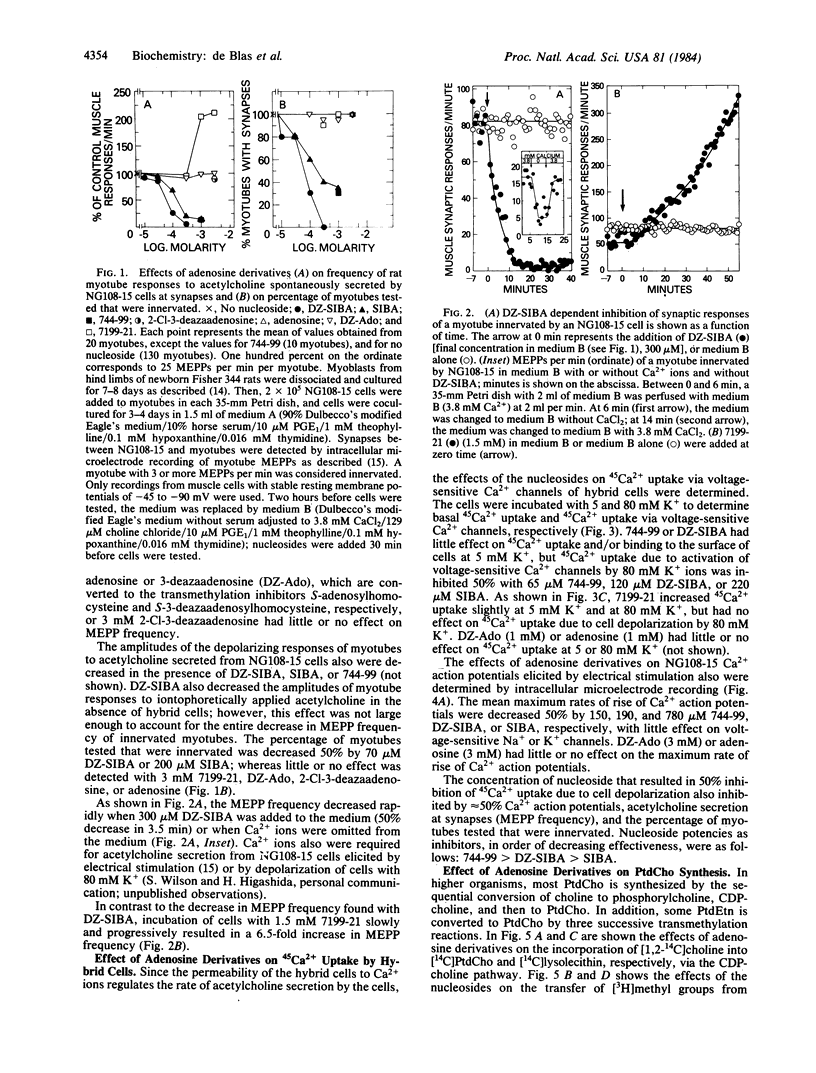
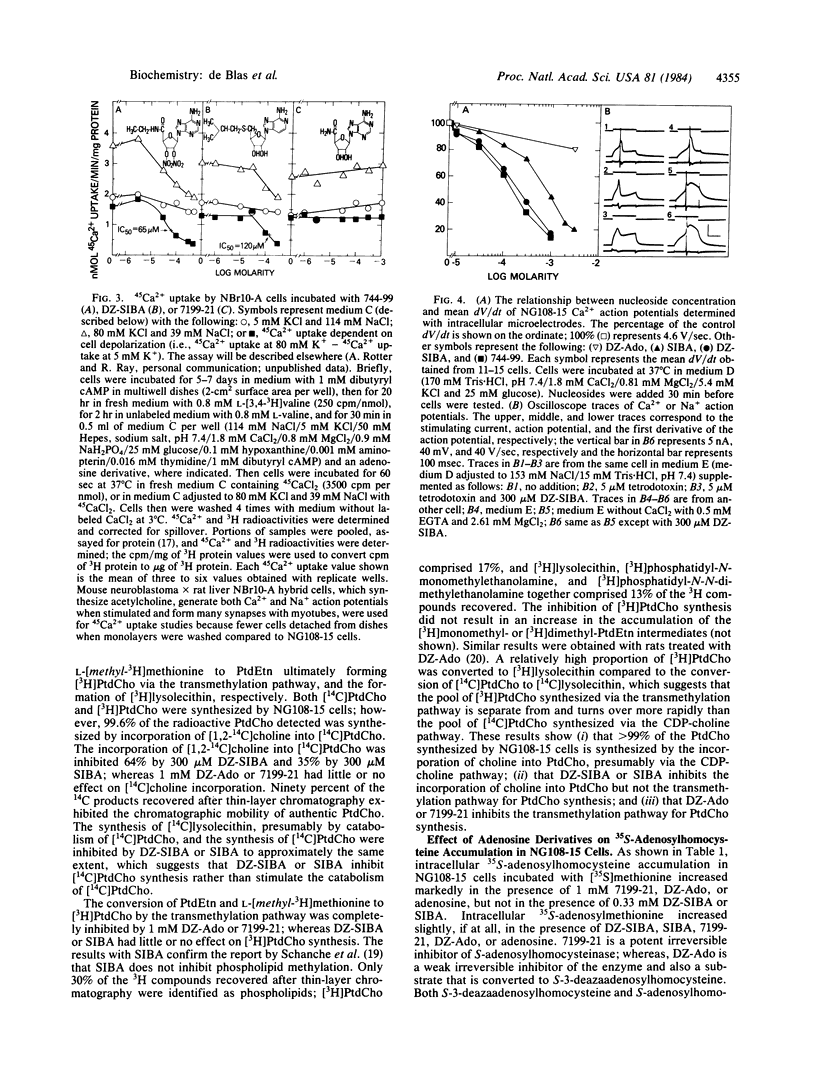
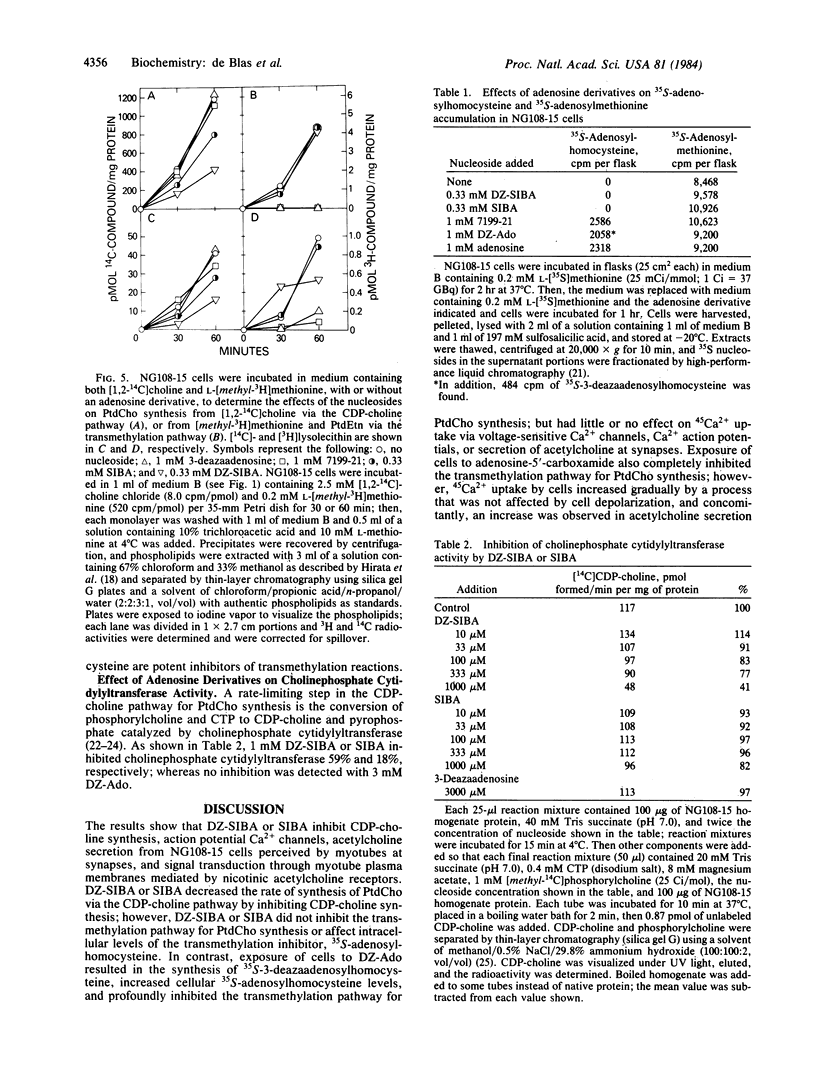
The effects of putative transmethylation inhibitors were tested on stimulus-secretion coupling and neurotransmitter secretion at synapses between neuroblastoma X glioma hybrid cells and myotubes. 5'-Deoxy-5'-isobutylthio-3-deazaadenosine or 5'-deoxy-5'-isobutylthioadenosine inhibited CDP-choline synthesis catalyzed by cholinephosphate cytidylyltransferase (CTP:cholinephosphate cytidylyltransferase, EC 2.7.7.15) and thereby decreased the rate of phosphatidylcholine synthesis from CDP-choline, but did not affect the transmethylation pathway for phosphatidylcholine synthesis. These compounds also inhibited 45Ca2+ uptake by hybrid cells mediated by voltage-sensitive Ca2+ channels, acetylcholine secretion at synapses, and signal transduction through cell membranes mediated by myotube nicotinic acetylcholine receptors. In contrast, 3-deazaadenosine or adenosine inhibited the transmethylation pathway for phosphatidylcholine synthesis, but had no effect on Ca2+ action potentials, acetylcholine secretion, or signal transduction through cell membranes mediated by nicotinic acetylcholine receptors. These results show that the stimulus-secretion coupling and secretion reactions studied are not dependent on phospholipid methylation and suggest that the activity of action potential Ca2+ channels and the rate of neurotransmitter secretion are functionally coupled to the rate of phosphatidylcholine synthesis via the CDP-choline pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksamit R. R., Falk W., Cantoni G. L. Inhibition of chemotaxis by S-3-deazaadenosylhomocysteine in a mouse macrophage cell line. J Biol Chem. 1982 Jan 25;257(2):621–625. [PubMed] [Google Scholar]
- Chiang P. K., Guranowski A., Segall J. E. Irreversible inhibition of S-adenosylhomocysteine hydrolase by nucleoside analogs. Arch Biochem Biophys. 1981 Mar;207(1):175–184. doi: 10.1016/0003-9861(81)90023-0. [DOI] [PubMed] [Google Scholar]
- Chiang P. K., Im Y. S., Cantoni G. L. Phospholipids biosynthesis by methylations and choline incorporation: effect of 3-deazaadenosine. Biochem Biophys Res Commun. 1980 May 14;94(1):174–181. doi: 10.1016/s0006-291x(80)80203-8. [DOI] [PubMed] [Google Scholar]
- Crews F. T., Morita Y., Hirata F., Axelrod J., Siraganian R. P. Phospholipid methylation affects immunoglobulin E-mediated histamine and arachidonic acid release in rat leukemia basophils. Biochem Biophys Res Commun. 1980 Mar 13;93(1):42–49. doi: 10.1016/s0006-291x(80)80243-9. [DOI] [PubMed] [Google Scholar]
- Domschke W., Keppler D., Bischoff E., Decker K. Cytosine nucleotides in liver. Hoppe Seylers Z Physiol Chem. 1971 Feb;352(2):275–279. doi: 10.1515/bchm2.1971.352.1.275. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J., Crews F. T. Concanavalin A stimulates phospholipid methylation and phosphatidylserine decarboxylation in rat mast cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4813–4816. doi: 10.1073/pnas.76.10.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Hirata F., Strittmatter W. J., Axelrod J. beta-Adrenergic receptor agonists increase phospholipid methylation, membrane fluidity, and beta-adrenergic receptor-adenylate cyclase coupling. Proc Natl Acad Sci U S A. 1979 Jan;76(1):368–372. doi: 10.1073/pnas.76.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Toyoshima S., Axelrod J., Waxdal M. J. Phospholipid methylation: a biochemical signal modulating lymphocyte mitogenesis. Proc Natl Acad Sci U S A. 1980 Feb;77(2):862–865. doi: 10.1073/pnas.77.2.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im Y. S., Chiang P. K., Cantoni G. L. Guanidoacetate methyltransferase. Purification and molecular properties. J Biol Chem. 1979 Nov 10;254(21):11047–11050. [PubMed] [Google Scholar]
- Infante J. P. Rate-limiting steps in the cytidine pathway for the synthesis of phosphatidylcholine and phosphatidylethanolamine. Biochem J. 1977 Dec 1;167(3):847–849. doi: 10.1042/bj1670847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Hirata F., Ishizaka K., Axelrod J. Stimulation of phospholipid methylation, Ca2+ influx, and histamine release by bridging of IgE receptors on rat mast cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1903–1906. doi: 10.1073/pnas.77.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandel P., Edel-Harth S. Free nucleotides in the rat brain during post-natal development. J Neurochem. 1966 Jul;13(7):591–595. doi: 10.1111/j.1471-4159.1966.tb11955.x. [DOI] [PubMed] [Google Scholar]
- McGee R., Simpson P., Christian C., Mata M., Nelson P., Nirenberg M. Regulation of acetylcholine release from neuroblastoma x glioma hybrid cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1314–1318. doi: 10.1073/pnas.75.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minna J., Glazer D., Nirenberg M. Genetic dissection of neural properties using somatic cell hybrids. Nat New Biol. 1972 Feb 23;235(60):225–231. doi: 10.1038/newbio235225a0. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Christian C. N., Daniels M. P., Henkart M., Bullock P., Mullinax D., Nirenberg M. Formation of synapses between cells of a neuroblastoma X glioma hybrid clone and mouse myotubes. Brain Res. 1978 May 26;147(2):245–259. doi: 10.1016/0006-8993(78)90838-7. [DOI] [PubMed] [Google Scholar]
- Nelson P., Christian C., Nirenberg M. Synapse formation between clonal neuroblastoma X glioma hybrid cells and striated muscle cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):123–127. doi: 10.1073/pnas.73.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Eisenbarth G. S., Thompson J. M., Nirenberg M. Synapse turnover: a mechanism for acquiring synaptic specificity. Proc Natl Acad Sci U S A. 1978 May;75(5):2281–2285. doi: 10.1073/pnas.75.5.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanche J. S., Schanche T., Ueland P. M. Inhibition of phospholipid methyltransferase(s) from rat liver plasma membranes by analogues of S-adenosylhomocysteine. Mol Pharmacol. 1981 Nov;20(3):631–636. [PubMed] [Google Scholar]
- Schlessinger J., Axelrod D., Koppel D. E., Webb W. W., Elson E. L. Lateral transport of a lipid probe and labeled proteins on a cell membrane. Science. 1977 Jan 21;195(4275):307–309. doi: 10.1126/science.556653. [DOI] [PubMed] [Google Scholar]
- Sleight R., Kent C. Regulation of phosphatidylcholine biosynthesis in cultured chick embryonic muscle treated with phospholipase C. J Biol Chem. 1980 Nov 25;255(22):10644–10650. [PubMed] [Google Scholar]
- Vance D. E., de Kruijff B. The possible functional significance of phosphatidylethanolamine methylation. Nature. 1980 Nov 20;288(5788):277–279. doi: 10.1038/288277a0. [DOI] [PubMed] [Google Scholar]
- Yavin E. Regulation of phospholipid metabolism in differentiating cells from rat brain cerebral hemispheres in culture. Patterns of acetylcholine phosphocholine, and choline phosphoglycerides labeling from (methyl-14C)choline. J Biol Chem. 1976 Mar 10;251(5):1392–1397. [PubMed] [Google Scholar]
- Yavin E., Zutra A. Translocation and turnover of phospholipid analogs in plasma membrane-derived vesicles from cell cultures. Biochim Biophys Acta. 1979 Jun 2;553(3):424–437. doi: 10.1016/0005-2736(79)90298-0. [DOI] [PubMed] [Google Scholar]
- Zelinski T. A., Savard J. D., Man R. Y., Choy P. C. Phosphatidylcholine biosynthesis in isolated hamster heart. J Biol Chem. 1980 Dec 10;255(23):11423–11428. [PubMed] [Google Scholar]


