Abstract
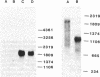
A cDNA of the human terminal deoxynucleotidyltransferase (TdT; "terminal transferase," EC 2.7.7.31) was isolated from a human lymphoblastoid cell cDNA library in lambda gt 11 by using immunological procedures. Four inserts containing 723 to 939 base pairs were recloned in pBR322 for hybridization and preliminary sequence studies. mRNA selected by hybridization to recombinant DNA was translated to a 58-kDa peptide that specifically immunoprecipitated with rabbit antibodies to calf terminal transferase and mouse monoclonal antibody to human terminal transferase. Blot hybridization of total poly(A)+ RNA from KM3 (TdT+) cells with nick-translated pBR322 recombinant DNA detected a message of about 2000 nucleotides, sufficient to code for the 580 amino acids in the protein. mRNA from terminal transferase- cells gave no signal in hybrid selection or RNA blot hybridization. The complete sequence of the 939-base-pair insert sequence was obtained from deletions cloned in pUC8. The DNA sequence contains an open reading frame coding for 238 amino acids, about 40% of the protein. Three peptides isolated by HPLC from tryptic digests of succinylated 58-kDa calf thymus terminal transferase were sequenced, providing 20, 18, and 22 residues of peptide sequence. A search of the translated sequence of the 939-base-pair insert shows three regions beginning after arginine that have greater than 90% homology with the sequence determined from the calf thymus terminal transferase peptides. These results provide unambiguous evidence that the human terminal transferase sequence has been cloned.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., Hutton J. J. Adenosine deaminase messenger RNAs in lymphoblast cell lines derived from leukemic patients and patients with hereditary adenosine deaminase deficiency. J Clin Invest. 1983 Jun;71(6):1649–1660. doi: 10.1172/JCI110920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bollum F. J. Antibody to terminal deoxynucleotidyl transferase. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4119–4122. doi: 10.1073/pnas.72.10.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollum F. J., Augl C., Chang L. M. Monoclonal antibodies to human terminal transferase. J Biol Chem. 1984 May 10;259(9):5848–5850. [PubMed] [Google Scholar]
- Bollum F. J., Chang L. M. Immunological detection of a conserved structure for terminal deoxynucleotidyltransferase. J Biol Chem. 1981 Aug 25;256(16):8767–8770. [PubMed] [Google Scholar]
- Bollum F. J. Terminal deoxynucleotidyl transferase as a hematopoietic cell marker. Blood. 1979 Dec;54(6):1203–1215. [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. V. Homogeneous terminal deoxynucleotidyl transferase. J Biol Chem. 1971 Feb 25;246(4):909–916. [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L. H., Wu R. New rapid methods for DNA sequencing based in exonuclease III digestion followed by repair synthesis. Nucleic Acids Res. 1982 Mar 25;10(6):2065–2084. doi: 10.1093/nar/10.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Janossy G., Bollum F. J., Bradstock K. F., McMichael A., Rapson N., Greaves M. F. Terminal transferase-positive human bone marrow cells exhibit the antigenic phenotype of common acute lymphoblastic leukemia. J Immunol. 1979 Oct;123(4):1525–1529. [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Hodnett J. L., Gray H. B., Jr Extracellular nucleases of pseudomonas BAL 31. III. Use of the double-strand deoxyriboexonuclease activity as the basis of a convenient method for the mapping of fragments of DNA produced by cleavage with restriction enzymes. Nucleic Acids Res. 1978 May;5(5):1445–1464. doi: 10.1093/nar/5.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- Nelson T., Brutlag D. Addition of homopolymers to the 3'-ends of duplex DNA with terminal transferase. Methods Enzymol. 1979;68:41–50. doi: 10.1016/0076-6879(79)68005-9. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Coleman P. F., Stark G. R. N-(Phosphonacetyl)-L-aspartate-resistant hamster cells overaccumulate a single mRNA coding for the multifunctional protein that catalyzes the first steps of UMP synthesis. J Biol Chem. 1979 Feb 10;254(3):974–980. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J. The use of exonuclease III for preparing single stranded DNA for use as a template in the chain terminator sequencing method. Nucleic Acids Res. 1979 Mar;6(3):831–848. doi: 10.1093/nar/6.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]