Abstract
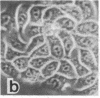
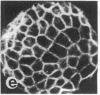
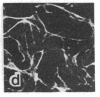
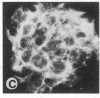
Tumor promoters such as phorbol 12-tetradecanoate 13-acetate (TPA), mezerein, teleocidin, aplysiatoxin, and benzoyl peroxide, although structurally unrelated, induce similar, profound changes in morphology in differentiated epithelial Madin-Darby canine kidney (MDCK) cell colonies. The alteration is evident in the organization of intermediate filaments in intact cells and in whole mounts of the nuclear matrix-intermediate filament (NM-IF) scaffold of the epithelial sheet. This substructure, obtained by salt extraction of the cytoskeletal framework, represents only 5% of the total cell protein but contains all of the intermediate filaments, nuclear matrix, and desmosomal core proteins arranged essentially as in the intact cell. The NM-IF is profoundly reorganized after exposure to TPA and retains the morphological changes observed in intact cells. These include bundling of the intermediate filaments, disruption of cell-cell borders, and marked deformation of the polygonal geometry of epithelia. Thus, TPA and all other complete or second-stage tumor promoters examined have a characteristic morphological signature that is not induced by mitogens, metabolic inhibitors, or agents known to disrupt microtubules or microfilaments. This signature, characteristic of tumor promoters, occurs in the absence of both protein and RNA synthesis. These results suggest that this response is prior to and independent of other biochemical markers for tumor promoters. Of the major filament systems, the cytokeratin network is implicated as an early or possibly primary site of tumor-promoter action because characteristics of the promoted cytoskeletal signature are observed in epithelial colonies after prior exposure to colchicine or cytochalasin D. Despite the massive reorganization of cytoskeletal morphology induced by TPA, the distribution of prelabeled proteins into structural fractions (i.e., cytoskeletal, chromatin, and the NM-IF) remains essentially unchanged. The sensitivity and specificity of the epithelial cell response suggest its possible use as a screen for promoting compounds.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsoum J., Varshavsky A. Mitogenic hormones and tumor promoters greatly increase the incidence of colony-forming cells bearing amplified dihydrofolate reductase genes. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5330–5334. doi: 10.1073/pnas.80.17.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Wendel E. J., Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop J., Toyama Y., Dlugosz A. A., Holtzer H. Selective effects of phorbol 12-myristate 13-acetate on myofibrils and 10-nm filaments. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5273–5277. doi: 10.1073/pnas.77.9.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. The effect of phorbol diesters on chicken embryo fibroblasts. Cancer Res. 1977 Sep;37(9):3257–3265. [PubMed] [Google Scholar]
- Fey E. G., Wan K. M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984 Jun;98(6):1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H., Mori M., Nakayasu M., Terada M., Sugimura T. A possible naturally occurring tumor promoter, teleocidin B from Streptomyces. Biochem Biophys Res Commun. 1979 Oct 12;90(3):976–983. doi: 10.1016/0006-291x(79)91923-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Epidermal growth factor, like phorbol esters, induces plasminogen activator in HeLa cells. Nature. 1978 Aug 17;274(5672):696–697. doi: 10.1038/274696a0. [DOI] [PubMed] [Google Scholar]
- Lichti U., Slaga T. J., Ben T., Patterson E., Hennings H., Yuspa S. H. Dissociation of tumor promoter-stimulated ornithine decarboxylase activity and DNA synthesis in mouse epidermis in vivo and in vitro by fluocinolone acetonide, a tumor-promotion inhibitor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3908–3912. doi: 10.1073/pnas.74.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks F., Bertsch S., Fürstenberger G. Ornithine decarboxylase activity, cell proliferation, and tumor promotion in mouse epidermis in vivo. Cancer Res. 1979 Oct;39(10):4183–4188. [PubMed] [Google Scholar]
- Marks F., Fürstenberger G., Kownatzki E. Prostaglandin E-mediated mitogenic stimulation of mouse epidermis in vivo by divalent cation ionophore A 23187 and by tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1981 Feb;41(2):696–702. [PubMed] [Google Scholar]
- Menko A. S., Toyama Y., Boettiger D., Holtzer H. Altered cell spreading in cytochalasin B: a possible role for intermediate filaments. Mol Cell Biol. 1983 Jan;3(1):113–125. doi: 10.1128/mcb.3.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi K., Levine L. alpha-Tocopherol inhibits 12-O-tetradecanoyl-phorbol-13-acetate-stimulated deacylation of cellular lipids, prostaglandin production, and changes in cell morphology of Madin-Darby canine kidney cells. Biochim Biophys Acta. 1980 Jul 14;619(1):11–19. doi: 10.1016/0005-2760(80)90238-6. [DOI] [PubMed] [Google Scholar]
- Ojakian G. K. Tumor promoter-induced changes in the permeability of epithelial cell tight junctions. Cell. 1981 Jan;23(1):95–103. doi: 10.1016/0092-8674(81)90274-9. [DOI] [PubMed] [Google Scholar]
- Parkinson E. K., Emmerson A. The effects of tumour promoters on the multiplication and morphology of cultured human epidermal keratinocytes. Carcinogenesis. 1982;3(5):525–531. doi: 10.1093/carcin/3.5.525. [DOI] [PubMed] [Google Scholar]
- Phaire-Washington L., Silverstein S. C., Wang E. Phorbol myristate acetate stimulates microtubule and 10-nm filament extension and lysosome redistribution in mouse macrophages. J Cell Biol. 1980 Aug;86(2):641–655. doi: 10.1083/jcb.86.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D. B., Crowe R. M., Pollack R. Tumor promoters induce changes in the chick embryo fibroblast cytoskeleton. Cell. 1979 Oct;18(2):361–368. doi: 10.1016/0092-8674(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Sivak A., Van Duuren B. L. Phenotypic expression of transformation: induction in cell culture by a phorbol ester. Science. 1967 Sep 22;157(3795):1443–1444. doi: 10.1126/science.157.3795.1443. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Fischer S. M., Nelson K., Gleason G. L. Studies on the mechanism of skin tumor promotion: evidence for several stages in promotion. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3659–3663. doi: 10.1073/pnas.77.6.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaga T. J., Klein-Szanto A. J., Triplett L. L., Yotti L. P., Trosko K. E. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981 Aug 28;213(4511):1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- Snoek G. T., Levine L. Requirements for protein synthesis and calcium for stimulation of prostaglandin synthesis in cultured rat liver cells by tumor promoters. Cancer Res. 1983 Oct;43(10):4743–4746. [PubMed] [Google Scholar]
- Sugimura T. Potent tumor promoters other than phorbol ester and their significance. Gan. 1982 Aug;73(4):499–507. [PubMed] [Google Scholar]
- Taketani Y., Oka T. Tumor promoter 12-O-tetradecanoylphorbol 13-acetate, like epidermal growth factor, stimulates cell proliferation and inhibits differentiation of mouse mammary epithelial cells in culture. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1646–1649. doi: 10.1073/pnas.80.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub M., Chuman L., Saier M. H., Jr, Sato G. Growth of Madin-Darby canine kidney epithelial cell (MDCK) line in hormone-supplemented, serum-free medium. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3338–3342. doi: 10.1073/pnas.76.7.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duuren B. L. Tumor-promoting agents in two-stage carcinogenesis. Prog Exp Tumor Res. 1969;11:31–68. doi: 10.1159/000391388. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B. Current concepts and controversies in chemical carcinogenesis. J Supramol Struct Cell Biochem. 1981;17(2):99–120. doi: 10.1002/jsscb.380170202. [DOI] [PubMed] [Google Scholar]
- Yang N. S., Kirkland W., Jorgensen T., Furmanski P. Absence of fibronectin and presence of plasminogen activator in both normal and malignant human mammary epithelial cells in culture. J Cell Biol. 1980 Jan;84(1):120–130. doi: 10.1083/jcb.84.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]