Abstract
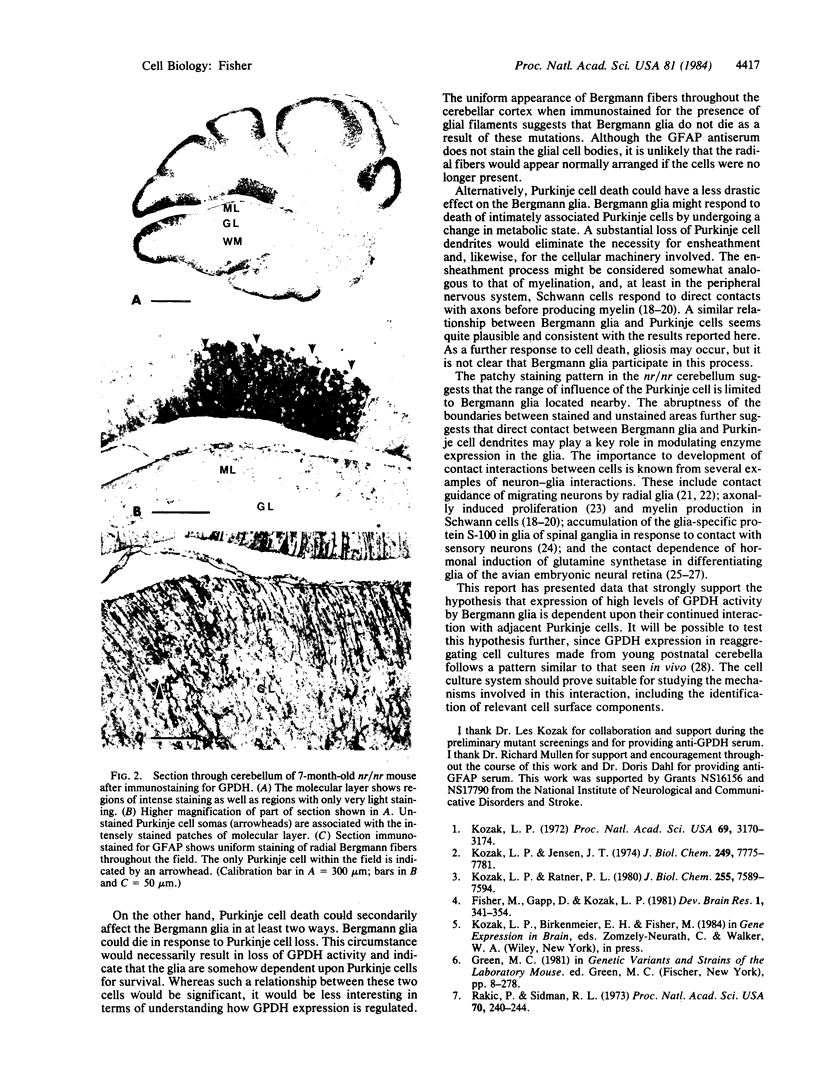
The developmentally regulated enzyme sn-glycerol-3-phosphate dehydrogenase (GPDH; EC 1.1.1.8) is characteristically present in relatively high levels in mature Bergmann glia of the mouse cerebellum. Preliminary studies identified several neurological mouse mutants with reduced glial enzyme activity. Immunohistochemical examination of GPDH expression in three mutants (lurcher, nervous, and Purkinje cell degeneration) revealed a positive correlation between glial enzyme expression and Purkinje cell presence. Whereas GPDH immunoreactivity appears normal in Bergmann glia from all three mutants at early times, immunoreactivity diminishes fairly rapidly after Purkinje cell loss, first in the Bergmann glia somas and then in the processes. Loss of immunoreactivity is uniform throughout the cerebellar cortex in lurcher and Purkinje cell degeneration where the entire Purkinje cell populations die. In nervous mice, in which some Purkinje cells survive, GPDH immunoreactivity is patchy throughout the cortex; it is present only where Purkinje cells remain. In contrast, Bergmann fibers appear uniformly distributed throughout the cortex of mutant cerebella, as demonstrated by immunostaining for the presence of glial filaments. This observation suggest that the loss of GPDH immunoreactivity is not a result of glial cell death. These results support the idea that GPDH expression in Bergmann glia depends upon their sustained interaction with adjacent Purkinje cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A. J., Charron L., Bray G. M. Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and radiographic study. J Neurocytol. 1976 Oct;5(8):565–573. doi: 10.1007/BF01175570. [DOI] [PubMed] [Google Scholar]
- Aguayo A. J., Epps J., Charron L., Bray G. M. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res. 1976 Mar 5;104(1):1–20. doi: 10.1016/0006-8993(76)90643-0. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. The development of Bergmann glia in mutant mice with cerebellar malformations: reeler, staggerer and weaver. Immunofluorescence study with antibodies to the glial fibrillary acidic protein. J Comp Neurol. 1974 May 15;155(2):219–229. doi: 10.1002/cne.901550207. [DOI] [PubMed] [Google Scholar]
- Caddy K. W., Biscoe T. J. Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1979 Oct 11;287(1020):167–201. doi: 10.1098/rstb.1979.0055. [DOI] [PubMed] [Google Scholar]
- Fisher M., Gapp D. A., Kozak L. P. Immunohistochemical localization of sn-glycerol-3-phosphate dehydrogenase in Bergmann glia and oligodendroglia in the mouse cerebellum. Brain Res. 1981 Jun;227(3):341–354. doi: 10.1016/0165-3806(81)90072-9. [DOI] [PubMed] [Google Scholar]
- Holton B., Weston J. A. Analysis of glial cell differentiation in peripheral nervous tissue. II. Neurons promote S 100 synthesis by purified glial precursor cell populations. Dev Biol. 1982 Jan;89(1):72–81. doi: 10.1016/0012-1606(82)90295-0. [DOI] [PubMed] [Google Scholar]
- Kozak L. P. Genetic control of -glycerolphosphate dehydrogenase in mouse brain. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3170–3174. doi: 10.1073/pnas.69.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak L. P., Jensen J. T. Genetic and developmental control of multiple forms of L-glycerol 3-phosphate dehydrogenase. J Biol Chem. 1974 Dec 25;249(24):7775–7781. [PubMed] [Google Scholar]
- Kozak L. P., Ratner P. L. Genetic regulation of translatable mRNA levels for mouse sn-glycerol-3-phosphate dehydrogenase during development of the cerebellum. J Biol Chem. 1980 Aug 25;255(16):7589–7594. [PubMed] [Google Scholar]
- Kozak L. P. The transition from embryonic to adult isozyme expression in reaggregating cell cultures of mouse brain. Dev Biol. 1977 Jan;55(1):160–169. doi: 10.1016/0012-1606(77)90327-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landis S. C. Ultrastructural changes in the mitochondria of cerebellar Purkinje cells of nervous mutant mice. J Cell Biol. 1973 Jun;57(3):782–797. doi: 10.1083/jcb.57.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Hormonal induction of glutamine synthetase in cultures of embryonic retina cells: requirement for neuron-glia contact interactions. Dev Biol. 1983 Apr;96(2):529–534. doi: 10.1016/0012-1606(83)90190-2. [DOI] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Induction of glutamine synthetase in embryonic neural retina: localization in Müller fibers and dependence on cell interactions. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6476–6480. doi: 10.1073/pnas.76.12.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen R. J., Eicher E. M., Sidman R. L. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci U S A. 1976 Jan;73(1):208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen R. J. Site of pcd gene action and Purkinje cell mosaicism in cerebella of chimaeric mice. Nature. 1977 Nov 17;270(5634):245–247. doi: 10.1038/270245a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Guidance of neurons migrating to the fetal monkey neocortex. Brain Res. 1971 Oct 29;33(2):471–476. doi: 10.1016/0006-8993(71)90119-3. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971 Mar;141(3):283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Weaver mutant mouse cerebellum: defective neuronal migration secondary to abnormality of Bergmann glia. Proc Natl Acad Sci U S A. 1973 Jan;70(1):240–244. doi: 10.1073/pnas.70.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J. L., Williams A. K., Glaser L., Bunge R. P. Studies of Schwann cell proliferation. II. Characterization of the stimulation and specificity of the response to a neurite membrane fraction. J Cell Biol. 1980 Mar;84(3):753–766. doi: 10.1083/jcb.84.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C., Triller A. Fate of presynaptic afferents to Purkinje cells in the adult nervous mutant mouse: a model to study presynaptic stabilization. Brain Res. 1979 Oct 12;175(1):11–36. doi: 10.1016/0006-8993(79)90511-0. [DOI] [PubMed] [Google Scholar]
- Weinberg H. J., Spencer P. S. Studies on the control of myelinogenesis. I. Myelination of regenerating axons after entry into a foreign unmyelinated nerve. J Neurocytol. 1975 Aug;4(4):395–418. doi: 10.1007/BF01261372. [DOI] [PubMed] [Google Scholar]
- Wetts R., Herrup K. Interaction of granule, Purkinje and inferior olivary neurons in lurcher chimaeric mice. I. Qualitative studies. J Embryol Exp Morphol. 1982 Apr;68:87–98. [PubMed] [Google Scholar]