Abstract
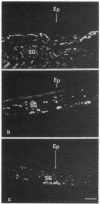
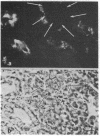
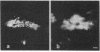
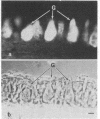
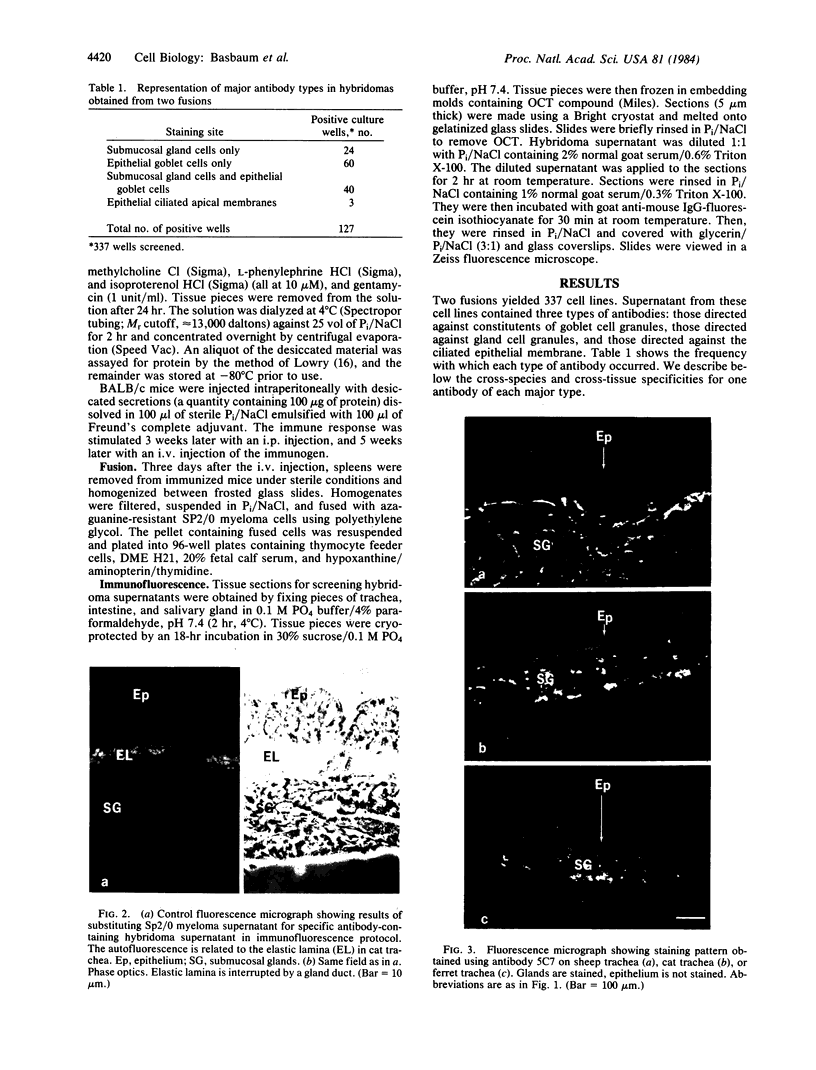
In the past, it has been difficult to identify the secretory product and control mechanisms associated with individual cell types making up mixed exocrine organs. This report establishes the feasibility of using immunological methods to characterize both the biochemical constituents and regulatory mechanisms associated with secretory cells in the trachea. Monoclonal antibodies directed against components of tracheal mucus were produced by immunizing mice with dialyzed, desiccated secretions harvested from tracheal organ culture. An immunofluorescence assay revealed that of the total 337 hybridomas screened, 100 produced antibodies recognizing goblet cell granules; 64, gland cell granules; and 3, antigen confined to the ciliated apical surface of the epithelium. The tracheal goblet cell antibody described in this report was strongly cross-reactive with intestinal goblet cells, as well as with a subpopulation of submandibular gland cells, but not with cells of Brunner's glands or the ciliated cell apical membrane. The serous cell antibody was not cross-reactive with goblet, Brunner's gland, or submandibular cells, or the ciliated cell apical membrane. The antibody directed against the apical membrane of ciliated cells did not cross-react with gland or goblet cells or the apical membrane of epithelial cells in the duodenum. Monoclonal antibodies, therefore, represent probes by which products unique to specific cells or parts of cells in the trachea can be distinguished. The antibodies, when used in enzyme immunoassays, can be used to quantitatively monitor secretion by individual cell types under a variety of physiological and pathological conditions. They also provide the means for purification and characterization of cell-specific products by immunoaffinity chromatography.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. J., Basbaum C. B. Mapping of adrenergic receptors in the trachea by autoradiography. Exp Lung Res. 1983 Nov;5(3):183–192. doi: 10.3109/01902148309061513. [DOI] [PubMed] [Google Scholar]
- Basbaum C. B., Ueki I., Brezina L., Nadel J. A. Tracheal submucosal gland serous cells stimulated in vitro with adrenergic and cholinergic agonists. A morphometric study. Cell Tissue Res. 1981;220(3):481–498. doi: 10.1007/BF00216752. [DOI] [PubMed] [Google Scholar]
- Boat T. F., Cheng P. W., Iyer R. N., Carlson D. M., Polony I. Human respiratory tract secretion. Mucous glycoproteins of nonpurulent tracheobronchial secretions, and sputum of patients with bronchitis and cystic fibrosis. Arch Biochem Biophys. 1976 Nov;177(1):95–104. doi: 10.1016/0003-9861(76)90419-7. [DOI] [PubMed] [Google Scholar]
- Boersma A., Lamblin G., Degand P., Roussel P. Separation of a complex mixture of oligosaccharides by HPLC on bonded-primary amine packing using a linear-gradient solvent system. Carbohydr Res. 1981 Aug 1;94(2):C7–C9. doi: 10.1016/s0008-6215(00)80723-5. [DOI] [PubMed] [Google Scholar]
- Butcher F. R., Putney J. W., Jr Regulation of parotid gland function by cyclic nucleotides and calcium. Adv Cyclic Nucleotide Res. 1980;13:215–249. [PubMed] [Google Scholar]
- Daniel P. F., Wolf G. Glycoprotein biosynthesis by organ cultures of hamster trachea. Biochim Biophys Acta. 1976 Nov 18;451(1):184–200. doi: 10.1016/0304-4165(76)90270-1. [DOI] [PubMed] [Google Scholar]
- Forstner J., Taichman N., Kalnins V., Forstner G. Intestinal goblet cell mucus: isolation and identification by immunofluorescence of a goblet cell glycoprotein. J Cell Sci. 1973 Mar;12(2):585–602. doi: 10.1242/jcs.12.2.585. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Kent P. W., Passatore M., Phipps R. J., Richardson P. S. The composition of tracheal mucus and the nervous control of its secretion in the cat. Proc R Soc Lond B Biol Sci. 1975 Dec 31;192(1106):49–76. doi: 10.1098/rspb.1975.0151. [DOI] [PubMed] [Google Scholar]
- German V. F., Ueki I. F., Nadel J. A. Micropipette measurement of airway submucosal gland secretion: laryngeal reflex. Am Rev Respir Dis. 1980 Sep;122(3):413–416. doi: 10.1164/arrd.1980.122.3.413. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb D., Reid L. Histochemical and autoradiographic investigation of the serous cells of the human bronchial glands. J Pathol. 1970 Feb;100(2):127–138. doi: 10.1002/path.1711000208. [DOI] [PubMed] [Google Scholar]
- Lamb D., Reid L. Histochemical types of acidic glycoprotein produced by mucous cells of the tracheobronchial glands in man. J Pathol. 1969 Aug;98(4):213–229. doi: 10.1002/path.1710980402. [DOI] [PubMed] [Google Scholar]
- Phipps R. J., Richardson P. S. The effects of irritation at various levels of the airway upon tracheal mucus secretion in the cat. J Physiol. 1976 Oct;261(3):563–581. doi: 10.1113/jphysiol.1976.sp011574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton P. M. Composition and control of secretions from tracheal bronchial submucosal glands. Nature. 1979 Jun 7;279(5713):551–552. doi: 10.1038/279551a0. [DOI] [PubMed] [Google Scholar]
- Quissell D. O., Lafferty J. L., Barzen K. A. Dispersed rat parotid cells: role of calcium and cAMP in the regulation of amylase secretion. J Dent Res. 1983 Feb;62(2):131–134. doi: 10.1177/00220345830620020901. [DOI] [PubMed] [Google Scholar]
- Roussel P., Degand P., Lamblin G., Laine A., Lafitte J. J. Biochemical definition of human tracheobronchial mucus. Lung. 1978;154(4):241–260. doi: 10.1007/BF02713541. [DOI] [PubMed] [Google Scholar]
- Sachdev G. P., Fox O. F., Wen G., Schroeder T., Elkins R. C., Carubelli R. Isolation and characterization of glycoproteins from canine tracheal mucus. Biochim Biophys Acta. 1978 Sep 26;536(1):184–196. doi: 10.1016/0005-2795(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Sachdev G. P., Myers F. J., Horton F. O., Fox O. F., Wen G., Rogers R. M., Carubelli R. Isolation, chemical composition, and properties of the major mucin component of normal human tracheobronchial secretions. Biochem Med. 1980 Aug;24(1):82–94. doi: 10.1016/0006-2944(80)90090-3. [DOI] [PubMed] [Google Scholar]
- Sherman J. M., Cheng P. W., Tandler B., Boat T. F. Mucous glycoproteins from cat tracheal goblet cells and mucous glands separated with EDTA. Am Rev Respir Dis. 1981 Oct;124(4):476–479. doi: 10.1164/arrd.1981.124.4.476. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Chakrin L. W., Wardell J. R., Jr, Kendrick W. Histochemistry of mucosubstances in the canine and human respiratory tract. Lab Invest. 1971 Dec;25(6):483–490. [PubMed] [Google Scholar]
- Tom-Moy M., Basbaum C. B., Nadel J. A. Localization and release of lysozyme from ferret trachea: effects of adrenergic and cholinergic drugs. Cell Tissue Res. 1983;228(3):549–562. doi: 10.1007/BF00211475. [DOI] [PubMed] [Google Scholar]
- Ueki I., German V. F., Nadel J. A. Micropipette measurement of airway submucosal gland secretion. Autonomic effects. Am Rev Respir Dis. 1980 Feb;121(2):351–357. doi: 10.1164/arrd.1980.121.2.351. [DOI] [PubMed] [Google Scholar]
- Williams J. A. Regulation of pancreatic acinar cell function by intracellular calcium. Am J Physiol. 1980 Apr;238(4):G269–G279. doi: 10.1152/ajpgi.1980.238.4.G269. [DOI] [PubMed] [Google Scholar]