Abstract
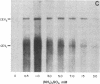
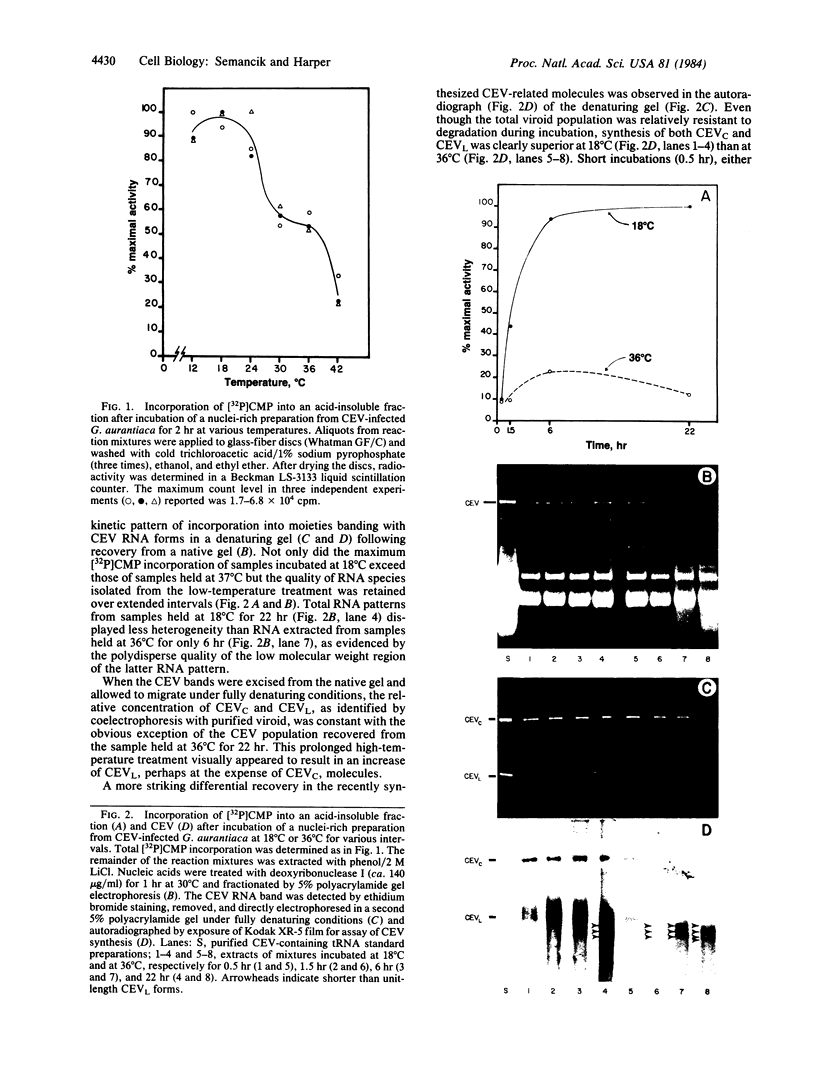
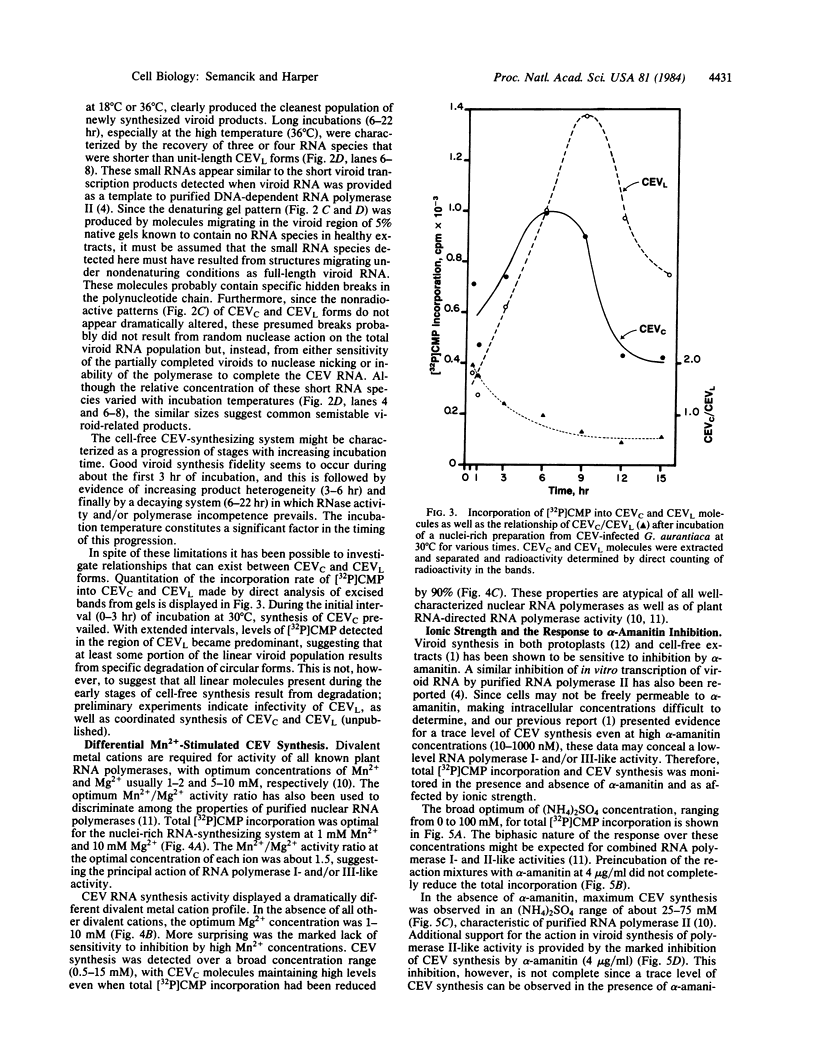
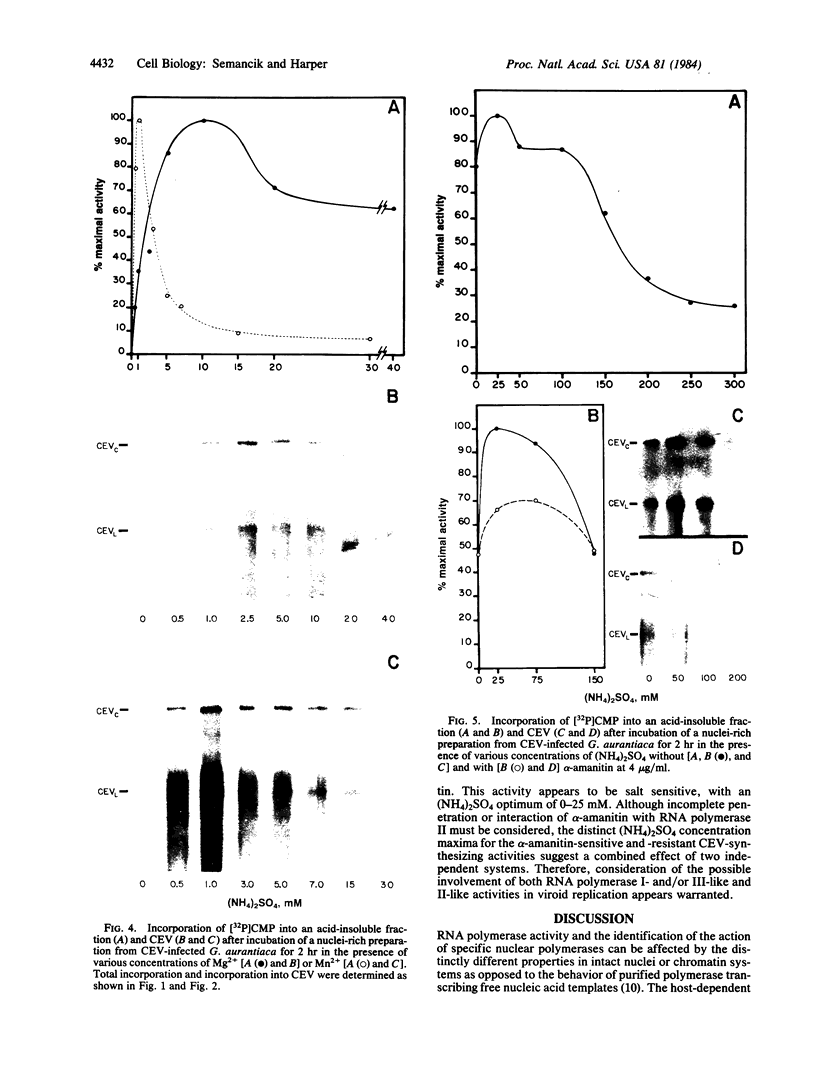
Cell-free synthesis of citrus exocortis viroid (CEV) in nuclei-rich preparations from infected Gynura aurantiaca was optimum at 18-24°C. Incubation of reaction mixtures at higher temperatures (30-36°C) resulted in an increase of CEV linear molecules and the recovery of incomplete or nicked newly synthesized RNA species. Although the Mg2+ optimum (2.5-5 mM) for CEV synthesis was lower than that for total [32P]CMP incorporation (10 mM), the response to Mn2+ ion was distinctly different. Whereas maximum total activity was observed in 1 mM Mn2+ with a pronounced reduction (80%) in 5 mM Mn2+, CEV synthesis was maintained in 1-15 mM Mn2+. Inhibition of α-amanitin-sensitive CEV synthesis in 200 mM (NH4)2SO4 resembles the reaction of RNA polymerase II on a free nucleic acid template. However, detection of trace levels of α-amanitin-resistant CEV synthesis activity inhibited by low (NH4)2SO4 concentrations (25 mM) suggests the possible involvement of RNA polymerase I- and/or III-like activity.
Keywords: nuclei-rich fraction, Mn2+ resistance, (NH4)2SO4 inhibition, α-amanitin, denaturing polyacrylamide gel electrophoresis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boege F., Rohde W., Sänger H. L. In vitro transcription of viroid RNA into full-length copies by RNA-dependent RNA polymerase from healthy tomato leaf tissue. Biosci Rep. 1982 Mar;2(3):185–194. doi: 10.1007/BF01116382. [DOI] [PubMed] [Google Scholar]
- Flores R., Semancik J. S. Properties of a cell-free system for synthesis of citrus exocortis viroid. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6285–6288. doi: 10.1073/pnas.79.20.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet J., Buhler J. M., Sentenac A., Fromageot P. Dissociation of two polypeptide chains from yeast RNA polymerase A. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3034–3038. doi: 10.1073/pnas.72.8.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrisak J. Purification and Subunit Structure of DNA-dependent RNA Polymerase III from Wheat Germ. Plant Physiol. 1981 Mar;67(3):438–444. doi: 10.1104/pp.67.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlbach H. P., Sänger H. L. Viroid replication is inhibited by alpha-amanitin. Nature. 1979 Mar 8;278(5700):185–188. doi: 10.1038/278185a0. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. Synthesis of RNA complementary to potato spindle tuber viroid using Q beta replicase. Virology. 1977 Jun 1;79(1):109–120. doi: 10.1016/0042-6822(77)90338-5. [DOI] [PubMed] [Google Scholar]
- Palmenberg A., Kaesberg P. Synthesis of complementary strands of heterologous RNAs with Qbeta replicase. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1371–1375. doi: 10.1073/pnas.71.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackwitz H. R., Rohde W., Sänger H. L. DNA-dependent RNA polymerase II of plant origin transcribes viroid RNA into full-length copies. Nature. 1981 May 28;291(5813):297–301. doi: 10.1038/291297a0. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Schumacher J., Sänger H. L., Riesner D. Subcellular localization of viroids in highly purified nuclei from tomato leaf tissue. EMBO J. 1983;2(9):1549–1555. doi: 10.1002/j.1460-2075.1983.tb01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Tsuruda D., Zaner L., Geelen J. L., Weathers J. G. Exocortis disease: subcellular distribution of pathogenic (viroid) RNA. Virology. 1976 Feb;69(2):669–676. doi: 10.1016/0042-6822(76)90495-5. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Avocado sunblotch viroid: primary sequence and proposed secondary structure. Nucleic Acids Res. 1981 Dec 11;9(23):6527–6537. doi: 10.1093/nar/9.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]