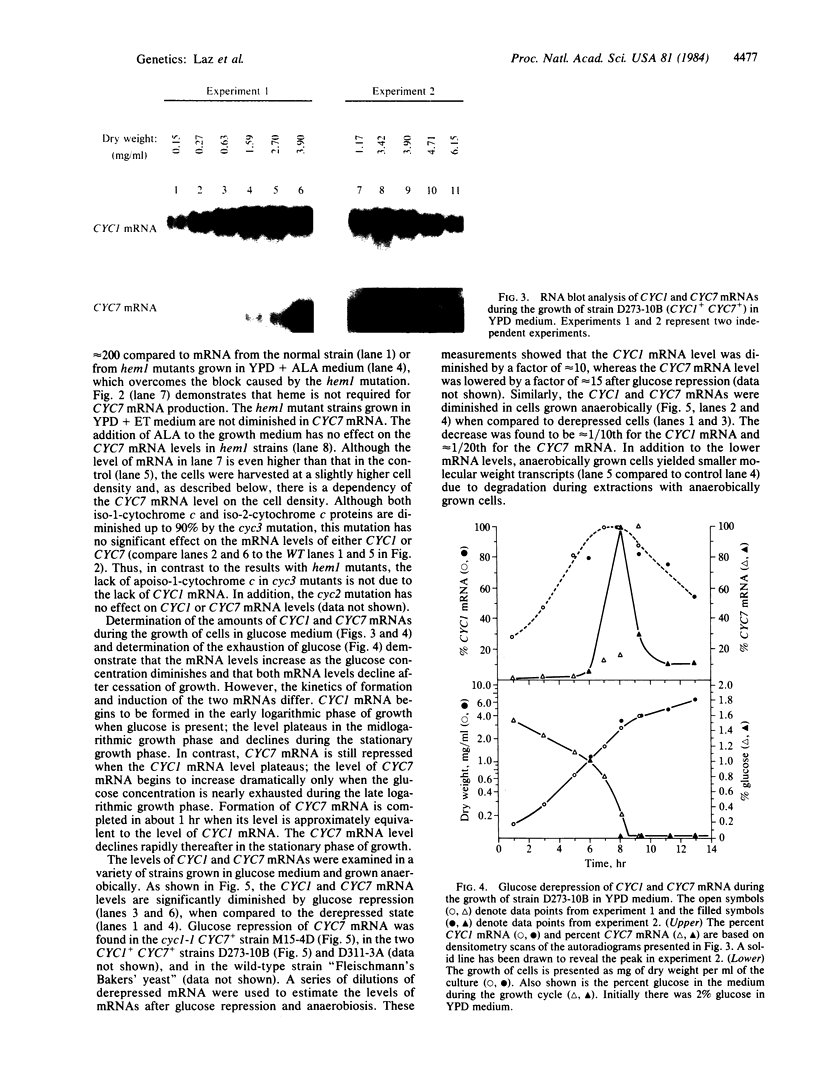
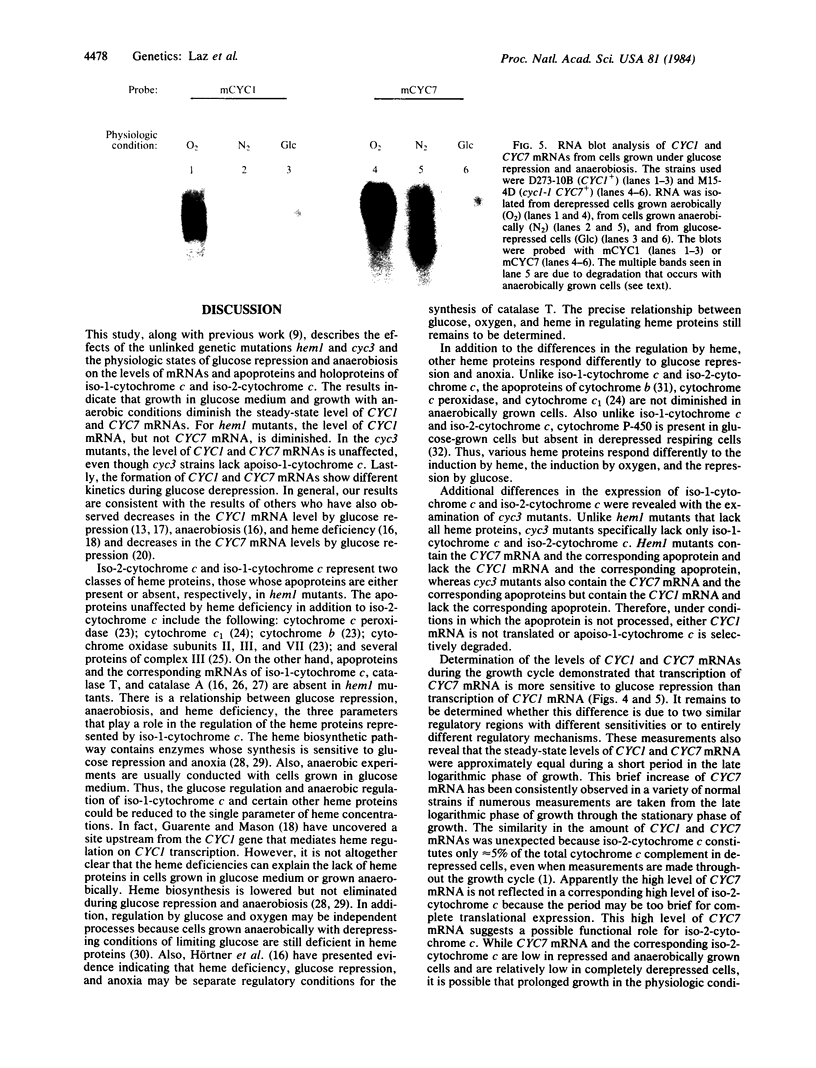
Abstract
The two unlinked genes CYC1 and CYC7 encode iso-1-cytochrome c and iso-2-cytochrome c, respectively, in the yeast Saccharomyces cerevisiae. An examination of the steady-state level of CYC1 and CYC7 mRNAs in normal and mutant strains grown under different conditions, along with previous results of apoprotein levels, demonstrate that CYC1 and CYC7 have similar and different modes of regulation. Both CYC1 and CYC7 mRNAs are diminished after anaerobic growth. In contrast, CYC1 mRNA but not CYC7 mRNA is decreased by heme deficiency in hem1 mutants. Although both CYC1 and CYC7 mRNAs are substantially lowered after growth in glucose medium, there is a difference in the kinetics of glucose derepression. CYC1 mRNA levels rise in the early logarithmic phase of growth before complete exhaustion of glucose, whereas CYC7 mRNA levels rise in the late logarithmic phase when the level of CYC1 mRNA has plateaued. For a brief period before cessation of growth, the level of CYC7 mRNA attains a level corresponding to the high derepressed level of CYC1 mRNA. The high amount of CYC7 mRNA is surprising because iso-2-cytochrome c constitutes only 5% of the total cytochrome c complement in derepressed cells. We suggest that iso-2-cytochrome c has the potential to comprise a major proportion of cytochrome c under certain physiologic conditions that have not been experimentally defined. The cyc3 mutant, which lacks the ability to attach heme groups to apocytochromes c, contains both CYC1 and CYC7 mRNAs in normal amounts. Yet, cyc3 mutants contain only apoiso-2-cytochrome c and not apoiso-1-cytochrome c. The lack of accumulation of apoiso-1-cytochrome c in cyc3 mutants, which contain CYC1 mRNA, suggests that apoiso-1-cytochrome c is extensively regulated by a post-transcriptional process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennetzen J. L., Hall B. D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J Biol Chem. 1982 Mar 25;257(6):3018–3025. [PubMed] [Google Scholar]
- Calvert A. F., Rodwell V. W. Metabolism of pipecolic acid in a Pseudomonas species. 3. L-alpha-aminoadipate delta-semialdehyde:nicotinamide adenine dinucleotide oxidoreductase. J Biol Chem. 1966 Jan 25;241(2):409–414. [PubMed] [Google Scholar]
- Clejan L., Beattie D. S., Gollub E. G., Liu K. P., Sprinson D. B. Synthesis of the apoprotein of cytochrome b in heme-deficient yeast cells. J Biol Chem. 1980 Feb 25;255(4):1312–1316. [PubMed] [Google Scholar]
- Denis C. L., Ferguson J., Young E. T. mRNA levels for the fermentative alcohol dehydrogenase of Saccharomyces cerevisiae decrease upon growth on a nonfermentable carbon source. J Biol Chem. 1983 Jan 25;258(2):1165–1171. [PubMed] [Google Scholar]
- Downie J. A., Stewart J. W., Brockman N., Schweingruber A. M., Sherman F. Structural gene for yeast iso-2-cytochrome c. J Mol Biol. 1977 Jun 25;113(2):369–384. doi: 10.1016/0022-2836(77)90147-4. [DOI] [PubMed] [Google Scholar]
- Gollub E. G., Liu K. P., Dayan J., Adlersberg M., Sprinson D. B. Yeast mutants deficient in heme biosynthesis and a heme mutant additionally blocked in cyclization of 2,3-oxidosqualene. J Biol Chem. 1977 May 10;252(9):2846–2854. [PubMed] [Google Scholar]
- Guarente L., Mason T. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell. 1983 Apr;32(4):1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- Guarente L., Ptashne M. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2199–2203. doi: 10.1073/pnas.78.4.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M. J., Holland J. P., Thill G. P., Jackson K. A. The primary structures of two yeast enolase genes. Homology between the 5' noncoding flanking regions of yeast enolase and glyceraldehyde-3-phosphate dehydrogenase genes. J Biol Chem. 1981 Feb 10;256(3):1385–1395. [PubMed] [Google Scholar]
- Hörtner H., Ammerer G., Hartter E., Hamilton B., Rytka J., Bilinski T., Ruis H. Regulation of synthesis of catalases and iso-1-cytochrome c in Saccharomyces cerevisiae by glucose, oxygen and heme. Eur J Biochem. 1982 Nov;128(1):179–184. doi: 10.1111/j.1432-1033.1982.tb06949.x. [DOI] [PubMed] [Google Scholar]
- Kärenlampi S. O., Marin E., Hänninen O. O. Effect of carbon source on the accumulation of cytochrome P-450 in the yeast Saccharomyces cerevisiae. Biochem J. 1981 Feb 15;194(2):407–413. doi: 10.1042/bj1940407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Sherman F., Jackson M., Gilmore R. A. Mapping and gene conversion studies with the structural gene for iso-1-cytochrome C in yeast. Genetics. 1975 Dec;81(4):615–629. doi: 10.1093/genetics/81.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. I., Gollub E. G., Beattie D. S. Synthesis of the proteins of complex III of the mitochondrial respiratory chain in heme-deficient cells. Eur J Biochem. 1982 Nov 15;128(2-3):309–313. doi: 10.1111/j.1432-1033.1982.tb06966.x. [DOI] [PubMed] [Google Scholar]
- Lowdon M. J., Gordon P. A., Stewart P. R. Regulation of the synthesis of mitochondrial enzymes and cytochromes. Distinction between catabolite repression and anaerobiosis in Saccharomyces cerevisiae. Arch Mikrobiol. 1972;85(4):355–361. doi: 10.1007/BF00549273. [DOI] [PubMed] [Google Scholar]
- Lowry C. V., Weiss J. L., Walthall D. A., Zitomer R. S. Modulator sequences mediate oxygen regulation of CYC1 and a neighboring gene in yeast. Proc Natl Acad Sci U S A. 1983 Jan;80(1):151–155. doi: 10.1073/pnas.80.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszkiewicz J., Biliński T. Effect of anaerobiosis and glucose on the content of haem and its precursors in intact yeast cells. Acta Biochim Pol. 1979;26(1-2):161–169. [PubMed] [Google Scholar]
- Matner R. R., Sherman F. Differential accumulation of two apo-iso-cytochromes c in processing mutants of yeast. J Biol Chem. 1982 Aug 25;257(16):9811–9821. [PubMed] [Google Scholar]
- McAlister L., Holland M. J. Targeted deletion of a yeast enolase structural gene. Identification and isolation of yeast enolase isozymes. J Biol Chem. 1982 Jun 25;257(12):7181–7188. [PubMed] [Google Scholar]
- McKnight G. L., Cardillo T. S., Sherman F. An extensive deletion causing overproduction of yeast iso-2-cytochrome c. Cell. 1981 Aug;25(2):409–419. doi: 10.1016/0092-8674(81)90059-3. [DOI] [PubMed] [Google Scholar]
- Montgomery D. L., Boss J. M., McAndrew S. J., Marr L., Walthall D. A., Zitomer R. S. The molecular characterization of three transcriptional mutations in the yeast iso-2-cytochrome c gene. J Biol Chem. 1982 Jul 10;257(13):7756–7761. [PubMed] [Google Scholar]
- Montgomery D. L., Leung D. W., Smith M., Shalit P., Faye G., Hall B. D. Isolation and sequence of the gene for iso-2-cytochrome c in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jan;77(1):541–545. doi: 10.1073/pnas.77.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K., Ammerer G., Hartter E., Ruis H. The effect of delta-aminolevulinate on catalase T-messenger RNA levels in delta-aminolevulinate synthase-defective mutants of Saccharomyces cerevisiae. J Biol Chem. 1980 Sep 10;255(17):8019–8022. [PubMed] [Google Scholar]
- Rogers D. T., Lemire J. M., Bostian K. A. Acid phosphatase polypeptides in Saccharomyces cerevisiae are encoded by a differentially regulated multigene family. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2157–2161. doi: 10.1073/pnas.79.7.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E., Schatz G. Cytochrome c1 of bakers' yeast. II. Synthesis on cytoplasmic robosomes and influence of oxygen and heme on accumulation of the apoprotein. J Biol Chem. 1976 Apr 10;251(7):1997–2004. [PubMed] [Google Scholar]
- Rothstein R. J., Sherman F. Genes affecting the expression of cytochrome c in yeast: genetic mapping and genetic interactions. Genetics. 1980 Apr;94(4):871–889. doi: 10.1093/genetics/94.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzgaber-Müller J., Schatz G. Heme is necessary for the accumulation and assembly of cytochrome c oxidase subunits in Saccharomyces cerevisiae. J Biol Chem. 1978 Jan 10;253(1):305–310. [PubMed] [Google Scholar]
- Sherman F., Stewart J. W. Genetics and biosynthesis of cytochrome c. Annu Rev Genet. 1971;5:257–296. doi: 10.1146/annurev.ge.05.120171.001353. [DOI] [PubMed] [Google Scholar]
- Sherman F., Stewart J. W., Helms C., Downie J. A. Chromosome mapping of the CYC7 gene determining yeast iso-2-cytochrome c: structural and regulatory regions. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1437–1441. doi: 10.1073/pnas.75.3.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., Stewart J. W., Margoliash E., Parker J., Campbell W. The structural gene for yeast cytochrome C. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1498–1504. doi: 10.1073/pnas.55.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Sherman F. Deletions of the iso-1-cytochrome c and adjacent genes of yeast: discovery of the OSM1 gene controlling osmotic sensitivity. Genetics. 1978 Aug;89(4):653–665. doi: 10.1093/genetics/89.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloszczuk W., Sprinson D. B., Ruis H. The relation of heme to catalase apoprotein synthesis in yeast. J Biol Chem. 1980 Mar 25;255(6):2624–2627. [PubMed] [Google Scholar]
- Woods R. A., Sanders H. K., Briquet M., Foury F., Drysdale B. E., Mattoon J. R. Regulation of mitochondrial biogenesis: enzymatic changes in cytochrome-deficient yeast mutants requiring delta-aminolevulinic acid. J Biol Chem. 1975 Dec 10;250(23):9090–9098. [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]
- Zitomer R. S., Hall B. D. Yeast cytochrome c messenger RNA. In vitro translation and specific immunoprecipitation of the CYC1 gene product. J Biol Chem. 1976 Oct 25;251(20):6320–6326. [PubMed] [Google Scholar]
- Zitomer R. S., Montgomery D. L., Nichols D. L., Hall B. D. Transcriptional regulation of the yeast cytochrome c gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3627–3631. doi: 10.1073/pnas.76.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]