Abstract
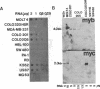
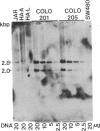
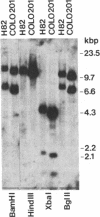
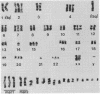
Two cell lines (COLO 201 and COLO 205) derived independently from a single adenocarcinoma of the human colon each harbored an approximately 10-fold amplification of the cellular oncogene c-myb and a proportional abundance of the 4-kilobase mRNA derived from c-myb. By contrast, expression of c-myb could not be detected in cells from a variety of other solid tumors, including other colon carcinomas. Analysis of the amplified DNA with restriction endonucleases failed to reveal any topographical abnormalities within c-myb. Neither COLO 201 nor COLO 205 carry the double minute chromosomes and homogeneously staining regions of chromosomes that frequently serve as karyotypic signatures of amplified DNA. Instead, amplified c-myb is carried on what appear to be disomic or trisomic copies of the same anomalous marker chromosome that is characteristic of both COLO 201 and COLO 205. The karyological origin of this abnormal chromosome is not presently apparent. Our findings show c-myb expression by cells outside of the hemopoietic lineage, raise the possibility that amplification and/or ectopic expression of c-myb may have contributed to the genesis of the tumor from which the cells of COLO 201 and COLO 205 arose, and suggest that amplification of cellular oncogenes may be a more common factor in tumorigenesis than might have been suspected from available karyological data.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Bishop J. M., Smith D. H., Chen E. Y., Colby W. W., Levinson A. D. Nucleotide sequence to the v-myc oncogene of avian retrovirus MC29. Proc Natl Acad Sci U S A. 1983 Jan;80(1):100–104. doi: 10.1073/pnas.80.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum J., Varshavsky A. Mitogenic hormones and tumor promoters greatly increase the incidence of colony-forming cells bearing amplified dihydrofolate reductase genes. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5330–5334. doi: 10.1073/pnas.80.17.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. C., Tlsty T. D., Schimke R. T. Enhancement of methotrexate resistance and dihydrofolate reductase gene amplification by treatment of mouse 3T6 cells with hydroxyurea. Mol Cell Biol. 1983 Jun;3(6):1097–1107. doi: 10.1128/mcb.3.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Groudine M. T. Rearrangement and amplification of c-abl sequences in the human chronic myelogenous leukemia cell line K-562. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4813–4817. doi: 10.1073/pnas.80.15.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S., Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982 Aug 12;298(5875):679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G., Wong-Staal F., Baluda M. A., Lengel C., Tronick S. R. Structural organization and expression of human DNA sequences related to the transforming gene of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7385–7389. doi: 10.1073/pnas.80.24.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Fanshier L., Bishop J. M., Moscovici C., Moscovici M. G. The genome and the intracellular RNAs of avian myeloblastosis virus. Cell. 1981 Jan;23(1):279–290. doi: 10.1016/0092-8674(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Franchini G., Love J., Simon M. I., Gallo R. C., Wong-Staal F. Chromosomal sublocalization of human c-myb and c-fes cellular onc genes. Nature. 1983 Jul 14;304(5922):169–171. doi: 10.1038/304169a0. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Johnston R. N., Beverley S. M., Schimke R. T. Rapid spontaneous dihydrofolate reductase gene amplification shown by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3711–3715. doi: 10.1073/pnas.80.12.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Kanda N., Schreck R. R., Bruns G., Latt S. A., Gilbert F., Alt F. W. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 1983 Dec;35(2 Pt 1):359–367. doi: 10.1016/0092-8674(83)90169-1. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Leprince D., Saule S., de Taisne C., Gegonne A., Begue A., Righi M., Stehelin D. The human DNA locus related to the oncogene myb of avian myeloblastosis virus (AMV): molecular cloning and structural characterization. EMBO J. 1983;2(7):1073–1078. doi: 10.1002/j.1460-2075.1983.tb01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. D., Nau M. M., Carney D. N., Gazdar A. F., Minna J. D. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983 Nov 10;306(5939):194–196. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- Moscovici M. G., Moscovici C. Isolation and characterization of a temperature-sensitive mutant of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1421–1425. doi: 10.1073/pnas.80.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushinski J. F., Potter M., Bauer S. R., Reddy E. P. DNA rearrangement and altered RNA expression of the c-myb oncogene in mouse plasmacytoid lymphosarcomas. Science. 1983 May 20;220(4599):795–798. doi: 10.1126/science.6687762. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature. 1983 Aug 18;304(5927):648–651. doi: 10.1038/304648a0. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Semple T. U., Quinn L. A., Woods L. K., Moore G. E. Tumor and lymphoid cell lines from a patient with carcinoma of the colon for a cytotoxicity model. Cancer Res. 1978 May;38(5):1345–1355. [PubMed] [Google Scholar]
- Ustacelebi S., Williams J. F. Temperature-sensitive mutants of adenovirus defective in interferon induction at non-permissive temperature. Nature. 1972 Jan 7;235(5332):52–53. doi: 10.1038/235052a0. [DOI] [PubMed] [Google Scholar]
- Vennström B., Fanshier L., Moscovici C., Bishop J. M. Molecular cloning of the avian erythroblastosis virus genome and recovery of oncogenic virus by transfection of chicken cells. J Virol. 1980 Nov;36(2):575–585. doi: 10.1128/jvi.36.2.575-585.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Moscovici C., Goodman H. M., Bishop J. M. Molecular cloning of the avian myelocytomatosis virus genome and recovery of infectious virus by transfection of chicken cells. J Virol. 1981 Aug;39(2):625–631. doi: 10.1128/jvi.39.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]