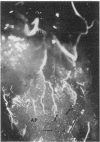
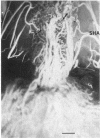
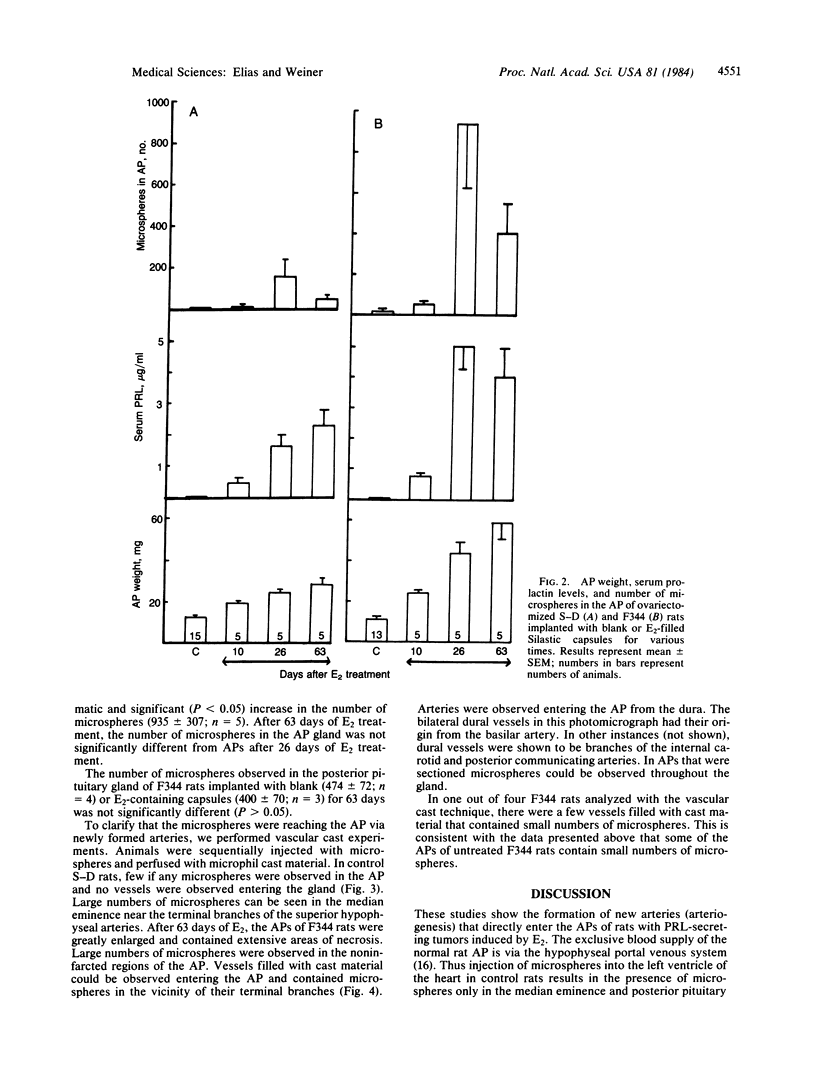
Abstract
The rat anterior pituitary gland (AP) receives all of its blood supply via the hypophyseal portal circulation. We now report, in rats with estradiol (E2)-induced prolactin-secreting tumors, that newly formed arteries directly supply the AP and that this arteriogenesis is closely correlated with the sensitivity of two strains of rats to the tumorigenic action of E2. Fischer 344 rats, a strain extremely sensitive to E2, and Sprague-Dawley rats, a less sensitive strain, were ovariectomized and implanted with E2-filled or empty Silastic capsules. Ten to 63 days later, microspheres (15 microns) were injected into the heart. Normally microspheres do not reach the AP because they are trapped in the primary portal capillary plexus. Some animals were also perfused with vascular cast material. In Fischer rats, after 63 days of E2, the pituitary weight, serum prolactin, and number of microspheres in the AP were 5-, 42-, and 18-fold greater than control values, respectively. The same parameters in E2-treated Sprague-Dawley rats were 2-, 27-, and 7-fold greater than control values. Vascular casts from E2-treated Fischer rats revealed numerous arteries entering the AP. No arteries to the AP were observed in Sprague-Dawley controls. These results show that E2-induced tumorigenesis of the AP is associated with the development of a direct arterial blood supply. We hypothesize that the regions supplied by these new arteries would receive systemic blood containing subphysiological concentrations of dopamine. The loss of dopaminergic inhibition in concert with E2 stimulation may lead to tumor formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbarino A., De Marinis L., Maira G., Menini E., Anile C. Serum prolactin response to thyrotropin-releasing hormone and metoclopramide in patients with prolactin-secreting tumors before and after transsphenoidal surgery. J Clin Endocrinol Metab. 1978 Nov;47(5):1148–1151. doi: 10.1210/jcem-47-5-1148. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N., Oliver C., Weiner H. J., Mical R. S., Porter J. C. Dopamine in hypophysial portal plasma of the rat during the estrous cycle and throughout pregnancy. Endocrinology. 1977 Feb;100(2):452–458. doi: 10.1210/endo-100-2-452. [DOI] [PubMed] [Google Scholar]
- CLIFTON K. H., MEYER R. K. Mechanism of anterior pituitary tumor induction by estrogen. Anat Rec. 1956 May;125(1):65–81. doi: 10.1002/ar.1091250106. [DOI] [PubMed] [Google Scholar]
- Costello R. T. Subclinical Adenoma of the Pituitary Gland. Am J Pathol. 1936 Mar;12(2):205–216.1. [PMC free article] [PubMed] [Google Scholar]
- Cronin M. J., Cheung C. Y., Weiner R. I., Goldsmith P. C. Mammotroph and gonadotroph volume percentage in the rat anterior pituitary after lesions of the medial basal hypothalamus. Neuroendocrinology. 1982 Feb;34(2):140–147. doi: 10.1159/000123291. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Prichard M. M. Studies of the hypothalamus and the pituitary gland with special reference to the effects of transection of the pituitary stalk. Acta Endocrinol Suppl (Copenh) 1975;201:1–216. [PubMed] [Google Scholar]
- De Nicola A. F., von Lawzewitsch I., Kaplan S. E., Libertun C. Biochemical and ultrastructural studies on estrogen-induced pituitary tumors in F344 rats. J Natl Cancer Inst. 1978 Sep;61(3):753–763. [PubMed] [Google Scholar]
- Eikenburg D. C., Ravitz A. J., Gudelsky G. A., Moore K. E. Effects of estrogen on prolactin and tuberoinfundibular dopaminergic neurons. J Neural Transm. 1977;40(4):235–244. doi: 10.1007/BF01257017. [DOI] [PubMed] [Google Scholar]
- Folkman J., Cotran R. Relation of vascular proliferation to tumor growth. Int Rev Exp Pathol. 1976;16:207–248. [PubMed] [Google Scholar]
- Gersten B. E., Baker B. L. Local action of intrahypophyseal implants of estrogen as revealed by staining with peroxidase-labeled antibody. Am J Anat. 1970 May;128(1):1–19. doi: 10.1002/aja.1001280102. [DOI] [PubMed] [Google Scholar]
- Greenblatt M., Shubi P. Tumor angiogenesis: transfilter diffusion studies in the hamster by the transparent chamber technique. J Natl Cancer Inst. 1968 Jul;41(1):111–124. [PubMed] [Google Scholar]
- Lloyd H. M., Meares J. D., Jacobi J. Effects of oestrogen and bromocryptine on in vivo secretion and mitosis in prolactin cells. Nature. 1975 Jun 5;255(5508):497–498. doi: 10.1038/255497a0. [DOI] [PubMed] [Google Scholar]
- MEYER R. K., CLIFTON K. H. Effect of diethylstilbestrol-induced tumorigenesis on the secretory activity of the rat anterior pituitary gland. Endocrinology. 1956 Jun;58(6):686–693. doi: 10.1210/endo-58-6-686. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Dopaminergic inhibition of prolactin synthesis and prolactin messenger RNA accumulation in cultured pituitary cells. J Biol Chem. 1980 Sep 10;255(17):8092–8097. [PubMed] [Google Scholar]
- Porter J. C., Barnea A., Cramer O. M., Parker C. R., Jr Hypothalamic peptide and catecholamine secretion: roles for portal and retrograde blood flow in the pituitary stalk in the release of hypothalamic dopamine and pituitary prolactin and LH. Clin Obstet Gynaecol. 1978 Aug;5(2):271–282. [PubMed] [Google Scholar]
- Sarkar D. K., Gottschall P. E., Meites J. Damage to hypothalamic dopaminergic neurons is associated with development of prolactin-secreting pituitary tumors. Science. 1982 Nov 12;218(4573):684–686. doi: 10.1126/science.7134966. [DOI] [PubMed] [Google Scholar]
- Schechter J. Ultrastructural changes in the capillary bed of human pituitary tumors. Am J Pathol. 1972 Apr;67(1):109–126. [PMC free article] [PubMed] [Google Scholar]
- Smythe G. A., Brandstater J. F. Oestrogen-induced hyperprolactinaemia in the rat: reduced concentrations of hypothalamic dopamine and the effects of bromocriptine. Aust J Biol Sci. 1980 Jun;33(3):329–339. doi: 10.1071/bi9800329. [DOI] [PubMed] [Google Scholar]
- Tiboldi T., Kurcz M., Kovács K. Examination of the blood supply of oestrogen hormone induced pituitary tumours in rats with India ink method. Neoplasma. 1968;15(3):259–265. [PubMed] [Google Scholar]
- Weiner R. I., Ganong W. F. Role of brain monoamines and histamine in regulation of anterior pituitary secretion. Physiol Rev. 1978 Oct;58(4):905–976. doi: 10.1152/physrev.1978.58.4.905. [DOI] [PubMed] [Google Scholar]
- Wiklund J., Wertz N., Gorski J. A comparison of estrogen effects on uterine and pituitary growth and prolactin synthesis in F344 and Holtzman rats. Endocrinology. 1981 Nov;109(5):1700–1707. doi: 10.1210/endo-109-5-1700. [DOI] [PubMed] [Google Scholar]