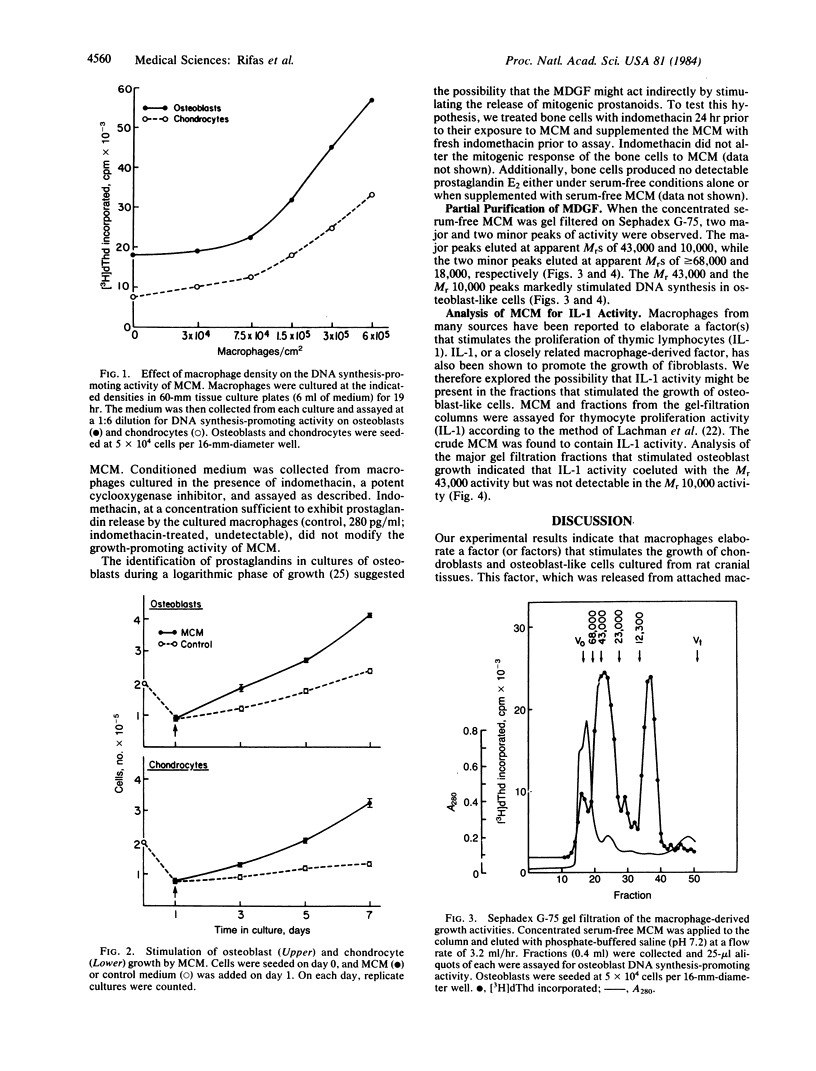
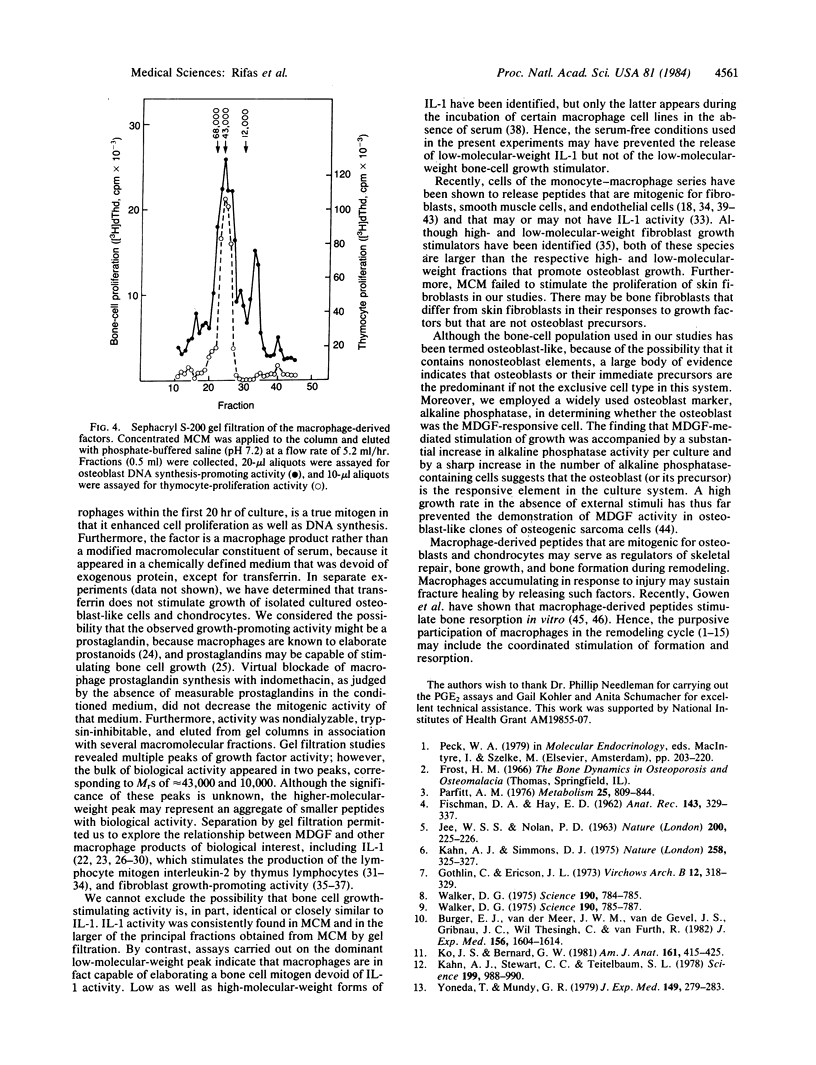
Abstract
Rat resident peritoneal macrophages in primary culture were found to elaborate a mitogenic factor (or factors) for rat osteoblast-like cells and chondrocytes but not for skin fibroblasts. Growth-promoting activity appeared in the incubation medium within the first 20 hr of macrophage culture and was released in amounts that paralleled the number of macrophages per culture. After their proliferative response, as judged by increases in DNA synthesis and cell number, the osteoblast-like cells became enriched in alkaline phosphatase, an index of osteoblast specialization. The macrophage-derived activity was nondialyzable and heat-stable, and it was eliminated by exposure to trypsin. Inhibition of prostaglandin cyclooxygenase failed to modify its generation. Partial purification (Amicon filter concentration, gel filtration) disclosed principal peaks of activity corresponding to Mr of 43,000 and 10,000. The crude conditioned medium and the Mr 43,000-peak, but not the low-molecular-weight peak, exhibited interleukin 1 activity, as judged by the ability to stimulate the proliferation of mouse thymic lymphocytes. The macrophage-derived growth factor described herein may participate in bone remodeling and repair and in primary bone and cartilage growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyden G., Handschumacher R. E. Purification and properties of human lymphocyte activating factor (LAF). J Immunol. 1977 May;118(5):1631–1638. [PubMed] [Google Scholar]
- Burger E. H., Van der Meer J. W., van de Gevel J. S., Gribnau J. C., Thesingh G. W., van Furth R. In vitro formation of osteoclasts from long-term cultures of bone marrow mononuclear phagocytes. J Exp Med. 1982 Dec 1;156(6):1604–1614. doi: 10.1084/jem.156.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. K., Peck W. A. Bone cells: a serum-free medium supports proliferation in primary culture. Science. 1978 Feb 3;199(4328):542–544. doi: 10.1126/science.564080. [DOI] [PubMed] [Google Scholar]
- FISCHMAN D. A., HAY E. D. Origin of osteoclasts from mononuclear leucocytes in regenerating newt limbs. Anat Rec. 1962 Aug;143:329–337. doi: 10.1002/ar.1091430402. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Mizel S. B., Fuller-Farrar J., Farrar W. L., Hilfiker M. L. Macrophage-independent activation of helper T cells. I. Production of Interleukin 2. J Immunol. 1980 Aug;125(2):793–798. [PubMed] [Google Scholar]
- Glenn K. C., Ross R. Human monocyte-derived growth factor(s) for mesenchymal cells: activation of secretion by endotoxin and concanavalin A. Cell. 1981 Sep;25(3):603–615. doi: 10.1016/0092-8674(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Gowen M., Meikle M. C., Reynolds J. J. Stimulation of bone resorption in vitro by a non-prostanoid factor released by human monocytes in culture. Biochim Biophys Acta. 1983 Jun 2;762(3):471–474. doi: 10.1016/0167-4889(83)90014-9. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Greenburg G. B., Hunt T. K. The proliferative response in vitro of vascular endothelial and smooth muscle cells exposed to wound fluids and macrophages. J Cell Physiol. 1978 Dec;97(3 Pt 1):353–360. doi: 10.1002/jcp.1040970310. [DOI] [PubMed] [Google Scholar]
- Göthlin G., Ericsson J. L. On the histogenesis of the cells in fracture callus. Electron microscopic autoradiographic observations in parabiotic rats and studies on labeled monocytes. Virchows Arch B Cell Pathol. 1973 Mar 30;12(4):318–329. [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- JEE W. S., NOLAN P. D. ORIGIN OF OSTEOCLASTS FROM THE FUSION OF PHAGOCYTES. Nature. 1963 Oct 19;200:225–226. doi: 10.1038/200225a0. [DOI] [PubMed] [Google Scholar]
- Kahn A. J., Simmons D. J. Investigation of cell lineage in bone using a chimaera of chick and quial embryonic tissue. Nature. 1975 Nov 27;258(5533):325–327. doi: 10.1038/258325a0. [DOI] [PubMed] [Google Scholar]
- Kahn A. J., Stewart C. C., Teitelbaum S. L. Contact-mediated bone resorption by human monocytes in vitro. Science. 1978 Mar 3;199(4332):988–990. doi: 10.1126/science.622581. [DOI] [PubMed] [Google Scholar]
- Ko J. S., Bernard G. W. Osteoclast formation in vitro from bone marrow mononuclear cells in osteoclast-free bone. Am J Anat. 1981 Aug;161(4):415–425. doi: 10.1002/aja.1001610407. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., WU M. L., HIXON W. S., CRAWFORD E. J. The quantitative histochemistry of brain. II. Enzyme measurements. J Biol Chem. 1954 Mar;207(1):19–37. [PubMed] [Google Scholar]
- Lachman L. B., Hacker M. P., Blyden G. T., Handschumacher R. E. Preparation of lymphocyte-activating factor from continuous murine macrophage cell lines. Cell Immunol. 1977 Dec;34(2):416–419. doi: 10.1016/0008-8749(77)90263-5. [DOI] [PubMed] [Google Scholar]
- Lachman L. B., Metzgar R. S. Characterization of high and low molecular weight lymphocyte-activating factor (interleukin I) from P388D and J774.1 mouse macrophage cell lines. J Reticuloendothel Soc. 1980 Jun;27(6):621–629. [PubMed] [Google Scholar]
- Lachman L. B., Page S. O., Metzgar R. S. Partial purification of human lymphocyte activating factor. Prep Biochem. 1980;10(4):387–403. doi: 10.1080/00327488008061739. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Iscove N. N., Coutinho A. Two distinct factors are required for induction of T-cell growth. Nature. 1980 Feb 14;283(5748):664–666. doi: 10.1038/283664a0. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J. Production of macrophage-dependent fibroblast-stimulating activity (M-FSA) by murine macrophages. Effects on BALBc 3T3 fibroblasts. Exp Cell Res. 1978 Apr;113(1):47–56. doi: 10.1016/0014-4827(78)90086-1. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Majeska R. J., Rodan S. B., Rodan G. A. Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology. 1980 Nov;107(5):1494–1503. doi: 10.1210/endo-107-5-1494. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosentreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by a macrophage cell line, P388D1. II. Biochemical characterization of LAF induced by activated T cells and LPS. J Immunol. 1978 May;120(5):1504–1508. [PubMed] [Google Scholar]
- Mizel S. B. Physicochemical characterization of lymphocyte-activating factor (LAF). J Immunol. 1979 Jun;122(6):2167–2172. [PubMed] [Google Scholar]
- Mizel S. B., Rosenstreich D. L., Oppenheim J. J. Phorbol myristic acetate stimulates LAF production by the macrophage cell line, P388D. Cell Immunol. 1978 Sep 15;40(1):230–235. doi: 10.1016/0008-8749(78)90330-1. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part I of IV parts: mechanisms of calcium transfer between blood and bone and their cellular basis: morphological and kinetic approaches to bone turnover. Metabolism. 1976 Jul;25(7):809–844. doi: 10.1016/0026-0495(76)90151-7. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Characterization of fibroblast proliferation factors elaborated by antigen- and mitogen-stimulated guinea pig lymph node cells: differentiation from lymphocyte-derived chemotactic factor for fibroblasts, lymphocyte mitogenic factor, and interleukin 1. Cell Immunol. 1982 Oct;73(1):169–178. doi: 10.1016/0008-8749(82)90445-2. [DOI] [PubMed] [Google Scholar]
- Reingold D. F., Watters K., Holmberg S., Needleman P. Differential biosynthesis of prostaglandins by hydronephrotic rabbit and cat kidneys. J Pharmacol Exp Ther. 1981 Mar;216(3):510–515. [PubMed] [Google Scholar]
- Rifas L., Uitto J., Memoli V. A., Kuettner K. E., Henry R. W., Peck W. A. Selective emergence of differentiated chondrocytes during serum-free culture of cells derived from fetal rat calvaria. J Cell Biol. 1982 Feb;92(2):493–504. doi: 10.1083/jcb.92.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Smith K. A., Gilbride K. J., Favata M. F. Lymphocyte activating factor promotes T-cell growth factor production by cloned murine lymphoma cells. Nature. 1980 Oct 30;287(5785):853–855. doi: 10.1038/287853a0. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van P. T., Vignery A., Baron R. Cellular kinetics of the bone remodeling sequence in the rat. Anat Rec. 1982 Apr;202(4):445–451. doi: 10.1002/ar.1092020403. [DOI] [PubMed] [Google Scholar]
- Tran Van P., Vignery A., Baron R. An electron-microscopic study of the bone-remodeling sequence in the rat. Cell Tissue Res. 1982;225(2):283–292. doi: 10.1007/BF00214682. [DOI] [PubMed] [Google Scholar]
- Walker D. G. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. 1975 Nov 21;190(4216):784–785. doi: 10.1126/science.1105786. [DOI] [PubMed] [Google Scholar]
- Walker D. G. Spleen cells transmit osteopetrosis in mice. Science. 1975 Nov 21;190(4216):785–787. doi: 10.1126/science.1198094. [DOI] [PubMed] [Google Scholar]
- Wharton W., Gillespie G. Y., Russell S. W., Pledger W. J. Mitogenic activity elaborated by macrophage-like cell lines acts as competence factor(s) for BALB/c 3T3 cells. J Cell Physiol. 1982 Jan;110(1):93–100. doi: 10.1002/jcp.1041100115. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Yoneda T., Mundy G. R. Prostaglandins are necessary for osteoclast-activating factor production by activated peripheral blood leukocytes. J Exp Med. 1979 Jan 1;149(1):279–283. doi: 10.1084/jem.149.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]


