Abstract
Owl monkey peripheral blood mononuclear cells were treated with phytohemagglutinin and expanded in interleukin 2 (IL-2)-containing medium. The cells were then exposed to Herpesvirus saimiri (HVS strain S295C)-infected owl monkey kidney monolayer cells. Four to 6 weeks later, the lymphocytes showed increased clumping and cell growth and the ability to grow in the absence of IL-2. Control lymphocyte cultures not exposed to HVS eventually died out at approximately 6-8 weeks, even in the presence of IL-2. Although infected lymphocytes grew continuously in the absence of IL-2, their growth was enhanced by addition of IL-2 to the cultures. Natural killer cell-like cytotoxicity and gamma-interferon release were also enhanced by IL-2. All cultures were positive for HVS antigens, infectious centers, or DNA. The reactivity of monoclonal antibodies to cell surface markers suggested that the resultant cell lines were comprised of activated T cells. The properties of the in vitro-transformed cells were similar to those of cells established from HVS-induced owl monkey tumors. Our results suggest that infection of T lymphocytes with HVS results in decreased dependence of T cells upon exogenous IL-2 for growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. L., Griffith R. L., Neubauer R. H., Rabin H. The effect of T cell growth factor on the cell cycle of primate T cells. J Immunol. 1982 Nov;129(5):1849–1853. [PubMed] [Google Scholar]
- Desrosiers R. C., Falk L. A. Herpesvirus saimiri strain variability. J Virol. 1982 Jul;43(1):352–356. doi: 10.1128/jvi.43.1.352-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
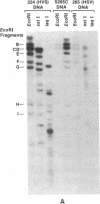
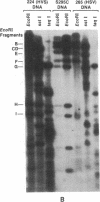
- Desrosiers R. C. Herpesvirus saimiri DNA in tumor cells--deleted sequences and sequence rearrangements. J Virol. 1981 Aug;39(2):497–509. doi: 10.1128/jvi.39.2.497-509.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. Specifically unmethylated cytidylic-guanylate sites in Herpesvirus saimiri DNA in tumor cells. J Virol. 1982 Aug;43(2):427–435. doi: 10.1128/jvi.43.2.427-435.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Stocks N., Zoon K., Stanton G. J., Timonen T., Herberman R. B. Positive self regulation of cytotoxicity in human natural killer cells by production of interferon upon exposure to influenza and herpes viruses. J Exp Med. 1982 Oct 1;156(4):1222–1234. doi: 10.1084/jem.156.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk L., Johnson D., Deinhardt F. Transformation of marmoset lymphocytes in vitro with Herpesvirus ateles. Int J Cancer. 1978 May 15;21(5):652–657. doi: 10.1002/ijc.2910210517. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B. Oncogenic herpesviruses of non-human primates. Biochim Biophys Acta. 1979 Nov 30;560(3):301–342. doi: 10.1016/0304-419x(79)90007-6. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Rabin H. Mechanisms of viral tumorigenesis. Adv Virus Res. 1982;27:281–334. doi: 10.1016/s0065-3527(08)60437-6. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Hewetson J. F., Hopkins R. F., 3rd, Sowder R. C., Neubauer R. H., Rabin H. A rapid, large scale purification procedure for gibbon interleukin 2. J Immunol. 1983 Aug;131(2):810–815. [PubMed] [Google Scholar]
- Hopkins R. F., 3rd, Witmer T. J., Neubauer R. H., Rabin H. Detection of antibodies to Epstein-Barr virus antigens by enzyme-linked immunosorbent assay. J Infect Dis. 1982 Dec;146(6):734–740. doi: 10.1093/infdis/146.6.734. [DOI] [PubMed] [Google Scholar]
- Johnson D. R., Jondal M. Herpesvirus ateles and herpesvirus saimiri transform marmoset T cells into continuously proliferating cell lines that can mediate natural killer cell-like cytotoxicity. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6391–6395. doi: 10.1073/pnas.78.10.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Werner F. J., Bauer I., Fleckenstein B. Structure of nonintegrated, circular Herpesvirus saimiri and Herpesvirus ateles genomes in tumor cell lines and in vitro-transformed cells. J Virol. 1982 Oct;44(1):295–310. doi: 10.1128/jvi.44.1.295-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Pearson G., Rabson A., Ablashi D. V., Falk L., Wolfe L., Dienhardt F., Rabin H. Antibody reactions to herpesvirus saimiri (HVS)-induced early and late antigens (EA and LA) in HVS-infected squirrel, marmoset and owl monkeys. Int J Cancer. 1973 Jul 15;12(1):270–289. doi: 10.1002/ijc.2910120128. [DOI] [PubMed] [Google Scholar]
- Markham P. D., Salahuddin S. Z., Kalyanaraman V. S., Popovic M., Sarin P., Gallo R. C. Infection and transformation of fresh human umbilical cord blood cells by multiple sources of human T-cell leukemia-lymphoma virus (HTLV). Int J Cancer. 1983 Apr 15;31(4):413–420. doi: 10.1002/ijc.2910310404. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Neubauer R. H., Briggs C. J., Noer K. B., Rabin H. Identification of normal and transformed lymphocyte subsets of nonhuman primates with monoclonal antibodies to human lymphocytes. J Immunol. 1983 Mar;130(3):1323–1329. [PubMed] [Google Scholar]
- Ortaldo J. R., Bonnard G. D., Herberman R. B. Cytotoxic reactivity of human lymphocytes cultured in vitro. J Immunol. 1977 Oct;119(4):1351–1357. [PubMed] [Google Scholar]
- Pearson G. R., Orr T., Rabin H., Cicmanec J., Ablashi D., Armstrong G. Antibody patterns to Herpesvirus saimiri-induced antigens in owl monkeys. J Natl Cancer Inst. 1973 Dec;51(6):1939–1943. doi: 10.1093/jnci/51.6.1939. [DOI] [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Rangan S. R., Gallagher R. E. Tumors and viruses in nonhuman primates. Adv Virus Res. 1979;24:1–123. doi: 10.1016/s0065-3527(08)60392-9. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Moss D. J., Wallace L. E., Rowe M., Misko I. S., Epstein M. A., Pope J. H. Long-term T-cell-mediated immunity to Epstein-Barr virus. Cancer Res. 1981 Nov;41(11 Pt 1):4216–4221. [PubMed] [Google Scholar]
- Rosén A., Persson K., Klein G. Human monoclonal antibodies to a genus-specific chlamydial antigen, produced by EBV-transformed B cells. J Immunol. 1983 Jun;130(6):2899–2902. [PubMed] [Google Scholar]
- Schirm S., Müller I., Desrosiers R. C., Fleckenstein B. Herpesvirus saimiri DNA in a lymphoid cell line established by in vitro transformation. J Virol. 1984 Mar;49(3):938–946. doi: 10.1128/jvi.49.3.938-946.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinitz M., Klein G., Koskimies S., Makel O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature. 1977 Sep 29;269(5627):420–422. doi: 10.1038/269420a0. [DOI] [PubMed] [Google Scholar]
- Wallen W. C., Neubauer R. H., Rabin H. In vitro immunological characteristics of lymphoid cells derived from owl monkeys infected with Herpesvirus saimiri. J Med Primatol. 1974;3(1):41–53. doi: 10.1159/000459963. [DOI] [PubMed] [Google Scholar]
- Wiranowska-Stewart M. Heterogeneity of human gamma interferon preparations: evidence for presence of alpha interferon. J Interferon Res. 1981 Feb;1(2):315–321. doi: 10.1089/jir.1981.1.315. [DOI] [PubMed] [Google Scholar]
- Wright J., Falk L. A., Deinhardt F. Interferon production by simian lymphoblastoid cell lines. J Natl Cancer Inst. 1974 Jul;53(1):271–275. doi: 10.1093/jnci/53.1.271. [DOI] [PubMed] [Google Scholar]