Abstract
Synthetic di- and oligopeptides are described that contain nucleophilic moieties attached to the α carbon of a glycine residue. These peptides are accepted by the peptide transport systems of Escherichia coli (and other microorganisms) and are capable of being hydrolyzed by intracellular peptidases. After liberation of its amino group the α-substituted glycine is chemically unstable (although it is stable in peptide form) and decomposes, releasing the nucleophilic moiety. Thus, the combined result of peptide transport and peptidase action is the intracellular release of the nucleophile. Peptides containing glycine residues α-substituted with thiophenol, aniline, or phenol are used as models for this type of peptide-assisted entry and their metabolism by E. coli is described. Peptides of this type have broad applicability to the study of microbial physiology and the development of an additional class of antimicrobial agents.
Keywords: peptide transport, portage transport, oligopeptide permease, antimicrobial agents, bacterial transport
Full text
PDF
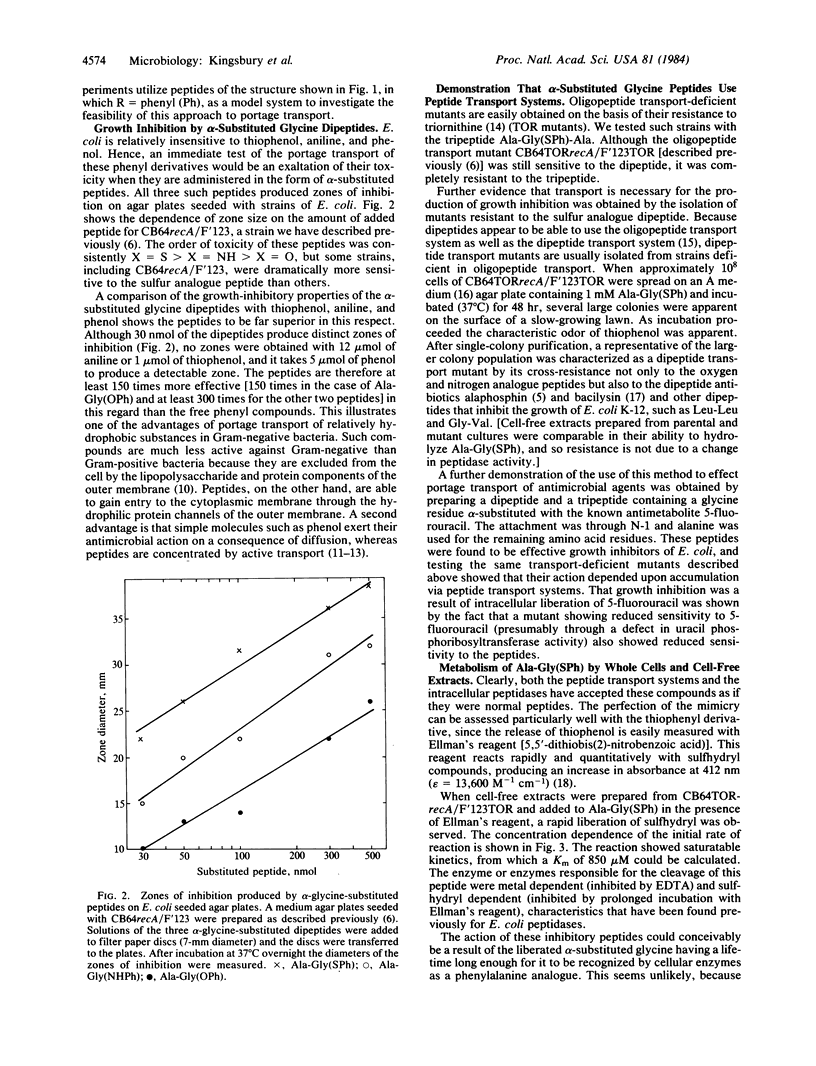
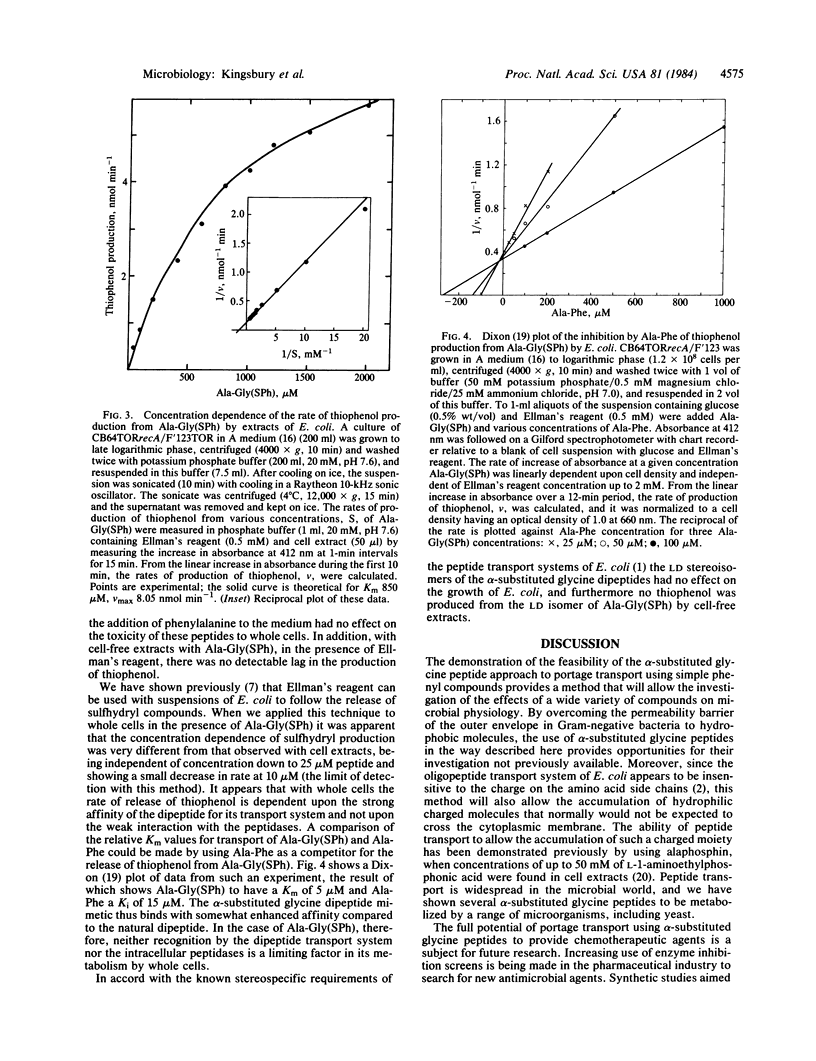

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. G., Atherton F. R., Hall M. J., Hassall C. H., Holmes S. W., Lambert R. W., Nisbet L. J., Ringrose P. S. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature. 1978 Mar 2;272(5648):56–58. doi: 10.1038/272056a0. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Ames G. F., Young J. D., Tsuchiya D., Lecocq J. Illicit transport: the oligopeptide permease. Proc Natl Acad Sci U S A. 1973 Feb;70(2):456–458. doi: 10.1073/pnas.70.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton F. R., Hall M. J., Hassall C. H., Lambert R. W., Ringrose P. S. Phosphonopeptides as antibacterial agents: rationale, chemistry, and structure-activity relationships. Antimicrob Agents Chemother. 1979 May;15(5):677–683. doi: 10.1128/aac.15.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Z., Gilvarg C. Triornithine-resistant strains of Escherichia coli. Isolation, definition, and genetic studies. J Biol Chem. 1974 Jan 10;249(1):143–148. [PubMed] [Google Scholar]
- Boehm J. C., Kingsbury W. D., Perry D., Gilvarg C. The use of cysteinyl peptides to effect portage transport of sulfhydryl-containing compounds in Escherichia coli. J Biol Chem. 1983 Dec 25;258(24):14850–14855. [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fickel T. E., Gilvarg C. Transport of impermeant substances in E. coli by way of oligopeptide permease. Nat New Biol. 1973 Feb 7;241(110):161–163. doi: 10.1038/newbio241161a0. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Becker J. M. Oligopeptide transport in proline peptidase mutants of Salmonella typhimurium. J Biol Chem. 1976 Sep 10;251(17):5300–5309. [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. On the distinction between peptidase activity and peptide transport. Biochim Biophys Acta. 1963 Jun 4;71:656–663. doi: 10.1016/0006-3002(63)91139-9. [DOI] [PubMed] [Google Scholar]
- Matthies D. Amidoalkylierung von Aminen mit alpha-Chlor-N-acylglycinestern. Pharmazie. 1970 Sep;25(9):522–524. [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Payne J. W., Bell G. Direct determination of the properties of peptide transport systems in Escherichia coli, using a fluorescent-labeling procedure. J Bacteriol. 1979 Jan;137(1):447–455. doi: 10.1128/jb.137.1.447-455.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. The role of the terminal carboxyl group on peptide transport in Escherichia coli. J Biol Chem. 1968 Jan 25;243(2):335–340. [PubMed] [Google Scholar]
- Perry D., Gilvarg C. Metabolism of alanylalanyl-S-[N-(2-thioethyl)]aminopyridine-2, 6-dicarboxylic acid]cysteine by suspensions of Escherichia coli. J Biol Chem. 1983 Dec 25;258(24):14856–14860. [PubMed] [Google Scholar]
- Walker J. E., Abraham E. P. The structure of bacilysin and other products of Bacillus subtilis. Biochem J. 1970 Jul;118(4):563–570. doi: 10.1042/bj1180563. [DOI] [PMC free article] [PubMed] [Google Scholar]



