Abstract
Background:
Aplastic anemia is a well-recognized form of marrow failure. The incidence of aplastic anemia is subjected to wide variation. Most cases are acquired and immune-mediated but there are also inherited forms.
Aim:
The study was conducted to assess the magnitude of the problem, morphological changes and determinants of aplastic anemia in North Bengal.
Materials and Methods:
A cross-sectional study had been conducted for a period of one year among 5 to 70 years age group. Initially complete blood count followed by bone marrow examination was done for diagnosis.
Results:
Out of 48 cases, 38 (79.17%) had hypocellular diagnosed as aplastic anemia, 5 (10.42%) each had normocellular and hypercellular bone marrow. Histopathology shows that 30 (78.95%) cases had increased iron stores in bone marrow and 8 (21.05%) cases had decreased iron stores. Subjects less than 20 years of age mostly (31.58%) suffered from non-severe disease in contrast to subjects in higher age groups who had severe or very severe disease, though the trend was not significantly different (χ2 for linear trend 0.171, P > 0.05).
Conclusion:
The study shows aplastic anemia is a common hematological abnormality among peripheral pancytopenia in North Bengal region where males were affected more than females.
Keywords: Aplastic anemia, non specific symptoms, north bengal region, peripheral pancytopenia
Introduction
Aplastic anemia is a potentially life-threatening failure of hemopoiesis characterized by pancytopenia and hypocellular bone marrow.[1,2] Aplastic anemia is defined as pancytopenia with a hypocellular bone marrow in the absence of an abnormal infiltrate and with no increase in reticulin. Most cases are acquired and immune-mediated but there are also inherited forms. Environmental triggers include drugs, viruses and toxins but most cases are idiopathic.[3]
Aplastic anemia represents a well-recognized form of marrow failure. The bone marrow failure implies that peripheral blood cytopenia has arisen primarily as a result of a specific failure of bone marrow precursor cells to produce mature cells, rather than the production of abnormal cells that have a shorten survival or the production of normal cells that are subjected to an abnormal environment. There are two groups of bone marrow failure: the aplastic anemia, in which the failure lies in the pluripotent stem cell, and the single-cell cytopenia, in which the failure lies in one of the committed cell lines. The annual incidence of aplastic anemia is about 2 cases per million population.[3] Aplastic anemia is 2-3 times more common in Asia than in the West. Acquired aplastic anemia most commonly presents between the ages of 15 and 25 years, but there is a second smaller peak in incidence after age 60 years. Certain histocompatibility locus specificities, especially HLA DR2, are associated with an underlying predisposition to acquired aplastic anemia. The incidence of aplastic anemia is subjected to wide variation throughout the world, the reason apparently lying in the environment rather than genetic factors, as people who move from the East to the West have the same incidence as the local population.[4] The present study, was conducted aiming to assess the magnitude of the problem, morphological changes and to study the determinants of aplastic anemia among the population of North Bengal region.
Materials and Methods
An institution-based cross-sectional study had been conducted for a period of one year from October 2008 to September 2009 in the Department of Pathology of North Bengal Medical College and Hospital, Darjeeling. Prior to that, necessary ethical approval was taken from the institutional ethical committee along with other departmental permission and informed consent from each and every participants. The institution is located in the Northern part of West Bengal, India serving the population as a tertiary care center and it is the only medical college covering the six districts of this state. The patients within the age group of 5 to 70 years whose blood samples were sent to the Department of Pathology of this Institution for routine examination and diagnosed as peripheral pancytopenia without hepatosplenomegaly, lymphadenopathy or bone pain were included in this study. The patients after a preliminary screening for peripheral pancytopenia were sent for radiological screening to the Department of Radiology of this institute to find out whether they had hepatosplenomegaly and/or abdominal and mediastinal lymphadenopathy. If the radiological investigations showed no hepatosplenomegaly and lymphadenopathy, the patients were finally included for this study. Initially complete blood count including total count RBC, WBC, platelet, hemoglobin estimation, reticulocyte count and red cell indices were performed followed by bone marrow examination by bone marrow trephine biopsy. Complete blood examination was done by automated cell counter in a system based on either aperture impedance or light scattering technology and also by visual direct methods using standard staining procedure in selected cases for quality assurance of automated cell counter.
Bone marrow trephine biopsy examination
In this study ‘Jamshidi Needle’ had been used. Posterior iliac spine was the site of choice for adults and the site for children was 2 cm below the tibial tuberosity. The preparation was done according to the standard procedure[5–7] and reticulin stain by Gordon and Sweet's method.[8] Interpretation of biopsy material was done according to the cellularity, comparison of the relative proportions of myeloid, erythroid and megakaryocytic cells, evaluation of focal infiltrative processes, other bone elements and reticulin. After detailed history-taking and clinical examination, laboratory investigations were done for inclusion of the patient in this study as pancytopenia according to the following criteria; i) Blood examination at the time of diagnosis-hemoglobin <10 gm/dl, total leukocytes count <4000/dl, platelet count <100,000/ cm. Decision for aplastic anemia was done by marrow cellularity i.e., decrease or loss of hematopoietic cell <30%, increased fat space, no malignant cell infiltration, no extensive marrow fibrosis, no extensive iron deposition, no evidence of malignant disease, myelofibrosis, storage disease or chemotherapy. Then the diagnosed cases of aplastic anemia were grouped as non-severe, severe and very severe according to the criteria as shown below.
-
Very Severe Aplastic Anemia
- As for severe aplastic anemia but neutrophils <0.2 × 109 /L.
-
Severe Aplastic Anemia
- BM cellularity <25%, or 25-50% with <30% residual hemopoietic cells
- Two out of three of the following: neutrophils <0.5 × 109 /L, platelets <20 × 109 /L, reticulocyte <20 × 109 /L.
-
Non-severe Aplastic Anemia
Patients not fulfilling the criteria for severe or very severe aplastic anemia
- With a hypocellular marrow.
- With two out of three of the following: neutrophils <1.5 × 109 /L, platelets <100 × 109 /L, hemoglobin <10 gm/dl.
A total of 48 cases with peripheral pancytopenia without hepatosplenomegaly and/or lymphadenopathy were included in this study.
Statistical analysis
The data were entered into an MS Excel sheet and analyzed using epi info, ver 7.0.9.34 en-US. The necessary descriptive statistics and chi-square for linear trend were applied for data analysis taking 95% confidence level as level of significance.
Results
Out of 48 cases of pancytopenia, 38 (79.17%) cases had hypocellular diagnosed as aplastic anemia, 5 cases (10.42%) had normocellular and 5 cases had hypercellular bone marrow of which 3 had erythroid hyperplasia and 2 had myeloid hyperplasia. Two cases out of total 5 cases with normocellular bone marrow had preponderance of mature eosinophils.
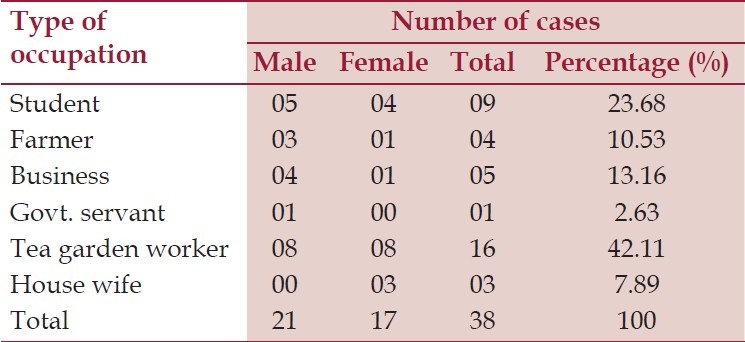
Among the 38 cases of aplastic anemia, 8 (21.05%), 13 (34-21%) and 17 (44.77%) had red blood cells count between 1-2 million/cu.mm, 2-3 million/cu.mm and (3-4 million/cu.mm), respectively. Most of the subjects (30 i.e. 78.95%) had hemoglobin more than 5 gm/dl and RBC count 3-4 million/cu.mm. Platelet count was below 20,000/cu.mm in majority of the (20 i.e. 52.63%) cases. Total count of white blood cells was 1000 to 2000/cu.mm in majority, only in 10 (26.32%) cases it was 3000 to 4000. Reticulocyte count increased in 5 (13.16%) cases. Out of total 38 cases with hypocellular marrow, 27 (71.05%) had normocytic-normochromic RBC, 8 cases (21.05%) had microcytic-hypochromic, and 3 cases (07.89%) had macrocytic red blood cells. All cases had low packed cell volume (100%). Out of 38 aplastic anemia cases, 6 cases (15.79%) had mild anisocytosis and poikilocytosis, 8 (21.05%) had moderate anisocytosis and poikilocytosis, 6 (15.79%) had severe anisocytosis and poikilocytosis in the form of teardrop cells, pencil-shaped cells, target cells etc., 13 (34.21%) cases had no significant abnormalities and 5 (13.16%) cases had fragmented RBC in peripheral blood film. Regarding residential status, 7 patients (4 male and 3 female) were coming from urban area and 31 patients (17 male and 14 female) were from rural area. Sixteen patients (42.11%) were tea garden workers as shown in Table 1.
Table 1.
Distribution of the study population according to their occupation, n = 38
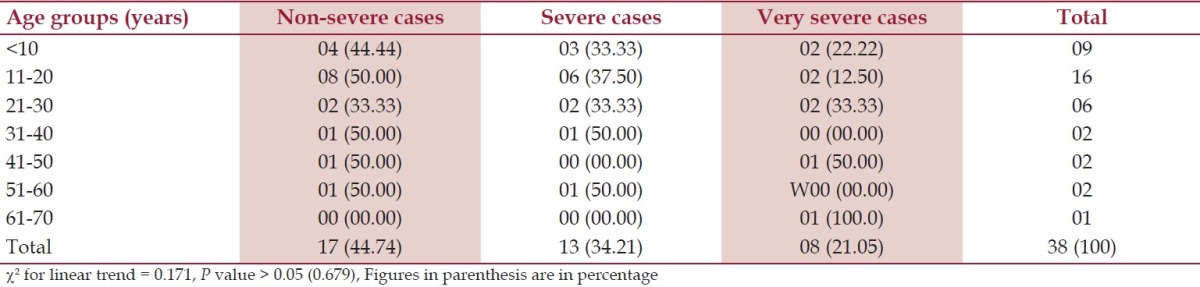
According to the symptoms, 35 (92.11%) were presented with weakness and 34 cases (89.47%) with lack of energy during household activities. Thirty-three cases (86.84%) had the evidence of pallor. Twenty-two cases (57.89%) were presented with the features of mucocutaneous bleeding, 18 cases (47.37%) had the history of excessive bleeding after cut injury, and out of total 10 females in the reproductive age group, history of excessive menstrual blood was the presenting feature in 7 cases (70%). Infection related to neutropenia was seen in 16 cases (42.11%) at the time of presentation. Symptoms related to anemia (weakness, lack of energy and pallor) and mucocutaneous bleeding were the commonest mode of presentation. In females of reproductive age group, excessive menstrual blood loss was the commonest presenting symptom. Histopathology showed that 30 (78.95%) cases had increased iron stores in bone marrow and 8 (21.05%) cases had decreased iron stores. Gordon and Sweet's stain showed reticulin positive in 5 (13.16%) cases, 33 (86.84%) cases had reticulin negative marrow. Out of 38 cases, 17 (44.74%) were in the non-severe group, 13 (34.21%) belonged to the severe and 8 (21.05%) were in very severe group according to sex. Females were affected more from severe and very severe diseases in comparison to male (χ2 = 5.59, df 1, P < 0.05) as shown in Table 2. Out of 38 cases, 2 (5.26%) cases showed normocellular marrow on examination of aspirated bone marrow smear, but aplastic anemia was confirmed after examination of trephine biopsy specimen. Microscopically the biopsy material showed a small nodular area (hot spots) consisting of all types of marrow cell, which in turn was surrounded by large fatty spaces as shown in Figure 1. In this study, cases of less than 20 years of age mostly (31.58%) had suffered from non-severe disease in contrast to higher age groups that had severe or very severe disease. The trend was not significantly different (χ2 for linear trend 0.171, P > 0.05) between non-severe, combined severe and very severe groups as shown in Table 3.
Table 2.
Degree of severity according to sex, n = 38
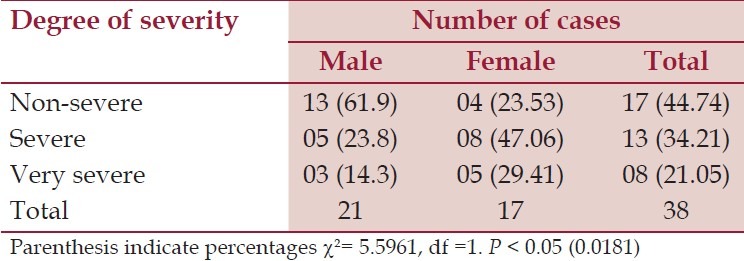
Figure 1.
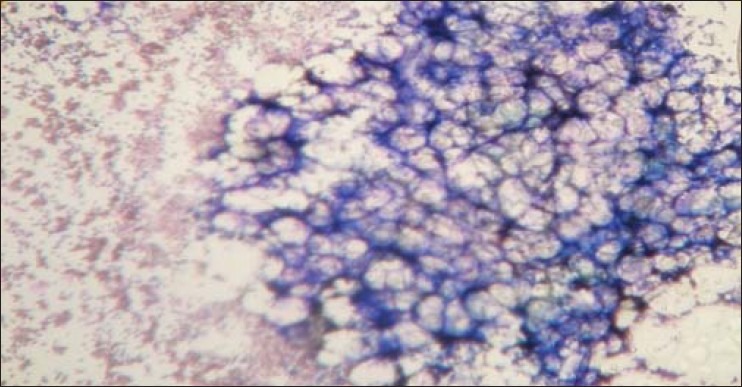
Hypocellular marrow with fat space (aspiration 10X, Leishman stain)Microscopically the aspiration material showed a small nodular area (“hot spots”) consisting of all types of marrow cell, which in turn was surrounded by large fatty spaces
Table 3.
Degree of severity according to the age, n = 38
Discussion
In this study, out of 48 cases, 38 cases (79.17%) had hypocellular bone marrow, 5 cases (10.42%) had normocellular and 5 (10.42%) cases had hypercellular bone marrow. So, it can be said that the majority of pancytopenia cases is due to aplastic anemia in this geographic area.
Reticulin is not increased and no abnormal cells are seen in aplastic anemia.[9] Here only 5 (13.16%) cases were reticulin positive.
Total count of RBC, WBC and platelets were lower than the normal value in the present study. Some patients (18.42%) had WBC count less than 1000, which not only has diagnostic utility but also disease categorization. The sex distribution of aplastic anemia in this study was found to be 1.25:1, male and female respectively reflecting male preponderance in the demographic profile or due to preferential reporting of the disease from the male counterpart of the society, especially from the rural areas, which constitutes 81.58% of the study group (aplastic anemia). The male-to-female ratio of the cases (1.6:1) differed from the almost equal ratio of the larger population of Turkey.[10] Sex distribution was 1.03 and 0.97 for males and females, respectively (χ2 = 0.272, P > .05) in a study from France. Greater prevalence of the disease found in rural areas may be either because ours is a referral hospital situated in a rural area. It is well documented that aplastic anemia is one of the causes of normocytic-normochromic anemia with some macrocytosis of remaining RBC.[4,5] Iron deficiency anemia is the most common cause of microcytic-hypochromic anemia in the developed and developing countries. So concomitant iron deficiency is the best explanation of the result obtained. In peripheral blood of aplastic anemia, RBC shows some degree of anisocytosis.[11] In the present study, 6 cases (15.79%) had mild anisocytosis and poikilocytosis, 8 cases (21.05%) had moderate anisocytosis and poikilocytosis, 6 cases (15.79%) had severe anisocytosis and poikilocytosis, 13 (34.21) cases had no significant abnormalities and 5 (13.16%) cases had fragmented RBC in peripheral blood film. These findings reflect that anemia is caused not only due to depressed production, but also some other causes like iron deficiency, peripheral destruction, Vitamin B12 and folic acid deficiency, etc. Regarding severity, in this study 44.74% patients were non-severe, 34.21% were severe and 21.05% were very severe. It was determined by absolute count of neutrophils, platelet count, and reticulocyte count, which is similar to the study in France.[12] It is well documented that bleeding and bruising secondary to thrombocytopenia is common and typical presentation. In this study, 92.11% and 89.47% patients presented with weakness and lack of energy, respectively followed by bleeding manifestations and infection.
Some patients with moderate disease by blood counts will have empty iliac crest biopsies, while “hot spots” of hematopoiesis may be seen in severe disease.[13] In this study, two such “hot spots” cases were identified. Morphologically each and every cell was absolutely normal except mildly megaloblastic erythropoiesis. Occurrence of “hot spots” in this study was 5.2%.
The use of various types of pesticides in the fields of agricultural industries are escalating day by day and there might have been some cause and effect relationship between indiscriminate use of newer pesticides and aplastic anemia.[14] In this study, out of total 38 aplastic anemia patients, 15 cases (39.48%) were tea garden workers and 4 cases (10.53%) were farmers. The tea garden workers are directly exposed to various insecticidal agents. Farmers are also exposed directly to varieties of pesticides. This is an indirect evidence that insecticidal agents might have some causal relationship with aplastic anemia. Further study is recommended.
Conclusion
The study shows aplastic anemia is a common hematological abnormality among peripheral pancytopenia in North Bengal region. Males were affected more than females in this study. Young rural population suffered more than the others. Most of the cases had hemoglobin level between 5-10 gm/dl, total count of white blood cells between 1000-2000/ cu. mm, platelet count <20,000/cu.mm, and marrow hypocellularity in bone marrow aspirated smear. Bone marrow aspiration from “hot spots” may lead to spurious finding of hypercellular marrow in case of aplastic anemia and bone marrow trephine biopsy is confirmatory in these cases. A large scale study is required to prove the hypothesis.
Acknowledgments
The authors thank all the faculty members of the Department of Community Medicine and Pathology of North Bengal Medical College.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of Aplastic Anaemia, British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 2.Bakhshi S, Abella E. Aplastic Anemia, eMedicine.com. 2009. (Accessed June 7, 2009, at: http:// emedicine.medscape.com/article/198759-overview. )
- 3.Brodsky RA, Jones RJ. Aplastic anaemia. Lancet. 2005;365:1647–56. doi: 10.1016/S0140-6736(05)66515-4. [DOI] [PubMed] [Google Scholar]
- 4.Hoffbrand VA, Catovsky D, Edward GD. Post graduate hematology. 5th ed. 350 main street, Malden, Massachusetts.02148-5020,USA: Blackwell Publishing. Inc; 2005. Tuddenham; pp. 176–206. [Google Scholar]
- 5.Lewis SM, Bain BJ, Bates I, editors. Practical Haematology. 9th ed. London: Churchill Livingstone; 2001. Dacie and Lewis; pp. 219–25. [Google Scholar]
- 6.U.S. National Library of Medicine and the National Institutes of Health. Medline Plus. Bone marrow aspiration. (Accessed October 28, 2005, at: http://www.nlm.nih.gov/medlineplus/ency /article/ 003658. htm. )
- 7. www.MedlinePlus Medical Encyclopedia Bone marrow biopsy. Available from (Accessed July 10, 2009, at http://www.nlm.nih.gov/medlineplus/ency/article/003934.htm. )
- 8.Bancroft JD, Gamble M. Theory and practice of histopathological technique. 5th ed. Canada: University of Toronto; 2001. [Google Scholar]
- 9.Marsh JC, Ball SE, Darbyshire P, Gordon-Smith EC, Keidan AJ, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2003;123:782–801. doi: 10.1046/j.1365-2141.2003.04721.x. [DOI] [PubMed] [Google Scholar]
- 10.Başlara Z, Aktuğlua G, Bolamanc Z, Büyükkeçecid F, Gezere S, Kansu E, et al. Incidence of aplastic anemia in Turkey: A hospital-based prospective multicentre study. Leuk Res. 1997;21:1135–9. doi: 10.1016/s0145-2126(97)00046-5. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman R, Benz EJ, Jr, Shattil SJ, Furie B, Cohen HJ, Silberstein LE. Hematology Basic Principle and Practice. 2nd ed. Churchill Livingstone; 1995. pp. 299–349. [Google Scholar]
- 12.Mary JY, Baumelou E, Guiguet M The French Cooperative Group for Epidemiological Study of Aplastic Anemia. Epidemiology of Aplastic Anemia in France: A Prospective Multicentric Study. Blood. 1990;75:1646–53. [PubMed] [Google Scholar]
- 13.Greer JP, Rodgers GM, Foerster J, Paraskevas F, Luken JN. Wintrobe's clinical hematology. 11th ed. Philadelphia: Lippincott Williams and Wilkins publishers; 2003. pp. 1397–413. [Google Scholar]
- 14.Maluf EM, Pasquini R, Eluf JN, Kelly J, Kaufman DW. Aplastic anemia in Brazil: Incidence and risk factors. Am J Hematol. 2002;7:268–74. doi: 10.1002/ajh.10232. [DOI] [PubMed] [Google Scholar]


