Abstract
Background:
The swine flu (H1N1) with rapid spread and panic in population is truly global pandemic, affected mainly younger population. There is need to accumulate evidence regarding patient's intensive care parameters for effective management of newer strains of influenza viral infections. Hence an observed retrospective record analysis of confirmed H1N1 patients admitted to intensive care unit (ICU) of a tertiary care centre is done.
Aims:
The study was designed to study the profile and pattern of H1N1 patients admitted to ICU and to study the distribution and associated factors with treatment outcomes.
Materials and Methods:
The demographic, clinical, and laboratory data of 32 (RT-PCR confirmed) H1N1cases were collected and analyzed using Fischer's exact test/paired t test between survivors and nonsurvivors to know their significance. This data included criteria for admission to ICU, type of lung injury, mode of oxygenation, antiviral, and other drugs used.
Results:
There were 11 males and 21 female. Age ranged from 19 to 72 years. Age group of 15–45 years had most cases (78%) and mortality (60%). Most common symptoms were fever and breathlessness (100%). The mean duration of breathlessness was statistically significant (P = 0.037) between two groups. Most common signs were tachycardia and tachypnea. The 75% cases developed acute respiratory distress syndrome (ARDS), of this 16% survived. Among these fatal cases nine were positive for procalcitonin (PCT) (P = 0.006). The rest of 25% developed acute lung injury (ALI) and recovered completely (P = 0.0001).
Conclusion:
Fever and breathlessness were the main presenting complaints. Tachypnea and tachycardia as clinical signs predict development of respiratory complications. Arterial blood gas analysis (ABG) and PaO2/FiO2 were important in deciding severity of lung injury and mode of ventilation. ARDS was observed to be the main cause of mortality in this study. Serum PCT level estimation is useful in determining outcome.
Keywords: Acute lung injury, Acute respiratory distress syndrome, Arterial blood gas analysis, H1N1, Intensive care unit
Introduction
The first case of novel strain of influenza A virus of swine origin (H1N1) was reported in Mexico during March 2009.[1] World health organization (WHO) declared a pandemic, with alert level of phase 6 within 3 months due to its global spread.[2] Most cases of H1N1 influenza infection present as mild or subclinical pneumonia, but some present as severe community acquired pneumonia (CAP) and require admission to intensive care unit (ICU) usually. The main reason for admission to ICU is due to acute lung injury/acute respiratory distress syndrome (ALI/ARDS). It causes primary viral pneumonia with secondary bacterial infections (20–30%) and it is notoriously known to affect younger population.[3–6] In India, this pandemic started from August 2009, with index case reported from Pune, and this epidemic spread to other parts of the country quickly.[7] There was reemergence of H1N1 during early 2012.
Pneumonia is one of the common and serious complications of H1N1.[8] The increased incidence of critical illness along with high transmissibility rate and rapid rise in the cases over a short period of time exerted significant stress on hospital resources, especially on intensive care.[9] There is need to accumulate evidence regarding patient's intensive care parameters for effective management of newer strains of influenza viral infections. Hence an observed retrospective record analysis of confirmed H1N1 patients admitted to ICU of a tertiary care centre, from August 2009 to April 2011, was undertaken with the following aims. The study was designed: to study the profile and pattern of H1N1 patients admitted to ICU and to study the distribution and associated factors (patient care factors and laboratory parameters) with treatment outcomes (survivors vs. nonsurvivors).
Materials and Methods
The present study had ethical clearance from institutional (MS Ramaiah) ethical committee. The present study is a retrospective study of successive, confirmed H1N1 patients with respiratory complication admitted to ICU of a tertiary care centre from August 2009 to April 2011. All 32 cases were RT-PCR confirmed at NIMHANS, Bangalore. Arterial blood gas analysis (ABG) was done at regular intervals to classify respiratory failure as either ARDS/ALI in accordance to American–European consensus conference.[10] The ratio of PaO2/FiO2 at the time of initiation of ventilatory support was noted. Of the 32 cases, 24 (75%) cases met ARDS criteria and 8 (25%) cases met ALI criteria. The demographic, clinical, laboratory and radiological data were collected from medical records and analyzed. The statistical analysis was done for all parameters for outcome among survivors versus nonsurvivors. All cases were treated with oral oseltamivir 75 mg bid, from the day of admission, in accordance with the WHO criteria.[11] All the patients were treated with broad spectrum antibiotics to cover coinfection/secondary bacterial infections and inotropic support for shock and ventilatory support given according to patient requirement. Serum procalcitonin (PCT), sputum, and blood culture were done to rule out bacterial sepsis at admission and at regular intervals. All other ICU patients, nurses, doctors, and other supporting staff were vaccinated and close contacts were treated prophylactically with oral oseltamivir according to WHO recommendations.
Statistical analysis
Descriptive statistics
Qualitative data like gender, morbidities, normal and abnormal laboratory values, and outcome were analyzed and presented as frequency and percentages. Quantitative data like age, laboratory values, and duration of hospital stay are presented as mean and standard deviation with 95% confidence intervals.
Analytical Statistics
To test association between clinical outcomes, laboratory values, hospital care management parameters the Independent ‘t’ test was applied. Fischer's exact test was applied to test association between clinical outcomes and qualitative variables. The test was considered significant at P < 0.05.
Results
It was observed that among the 1171 medical cases admitted to ICU during the study period, 17.9% (210/1171) were suspected to be suffering from flu, among them 2.7% (32/1171) cases were positive for H1N1. Proportion of positive cases among the suspected cases was 15.2% (32/210). Table 1 shows demography, comorbidities/risk factors and serum PCT value among the 32 H1N1 positive patients requiring intensive care. APACHE II score was calculated for all; it ranged from 9—18, and was not statistically significant between two groups. The male:female ratio was 1:1.9. The case fatality rate in males was 73.7% (8/11), in comparison to females with 57.2% (12/21) but is not statistically significant. The patient's age ranged from 19 to 72 years. Distribution of cases among age groups is shown in Figure 1, maximum number of cases and fatality were in the age group of 15–45 years, that is, 78.1% (25/32) and 60% (15/32), respectively. Most patients had fever (100%), breathlessness (100%), and cough (75%) as presenting complaints. The mean duration of breathlessness is statistically significant (P = 0.037) between two groups. The first case was reported in August 2009 and maximum number of cases and fatalities were reported during April to September 2010, that is, 65.6% (21/32) and 71.4% (15/21), respectively [Figure 2]. When cases with and without comorbidities were compared, outcome was not statistically significant (P = 0.431) [Table 1].
Table 1.
Demography, comorbities/risk factors and serum procalcitonin value among the cases
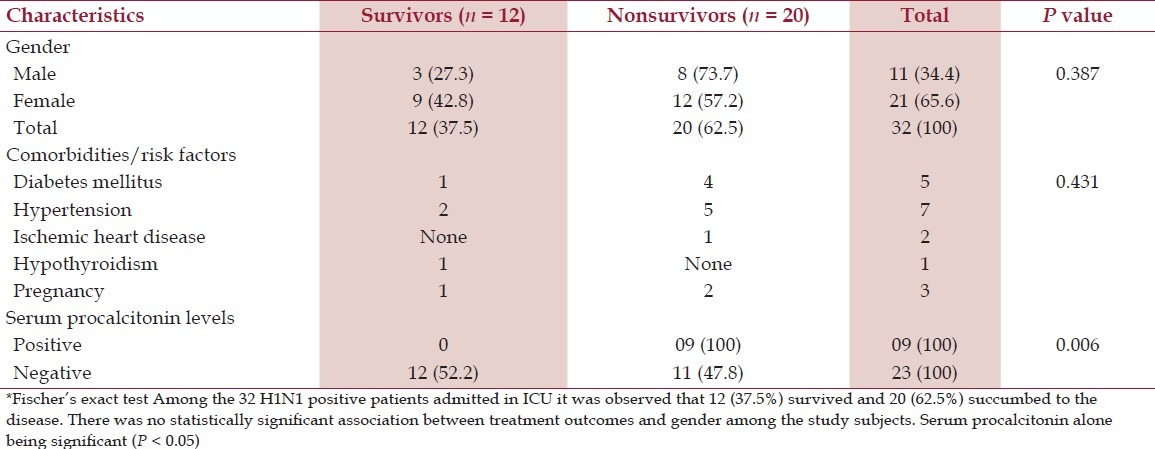
Figure 1.
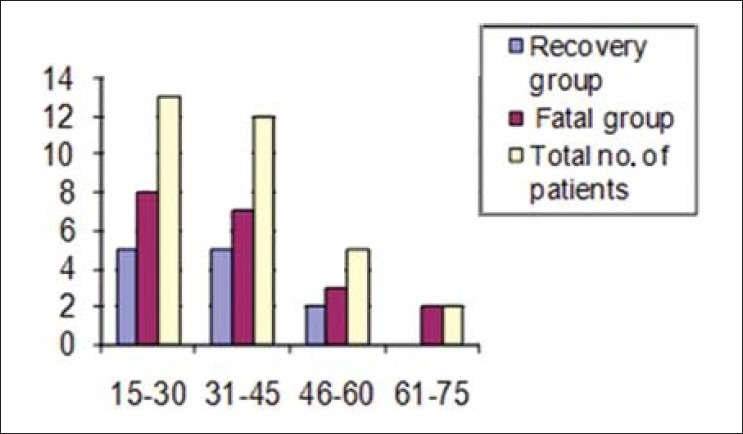
Bar diagram showing distribution of cases among different age groups
Figure 2.
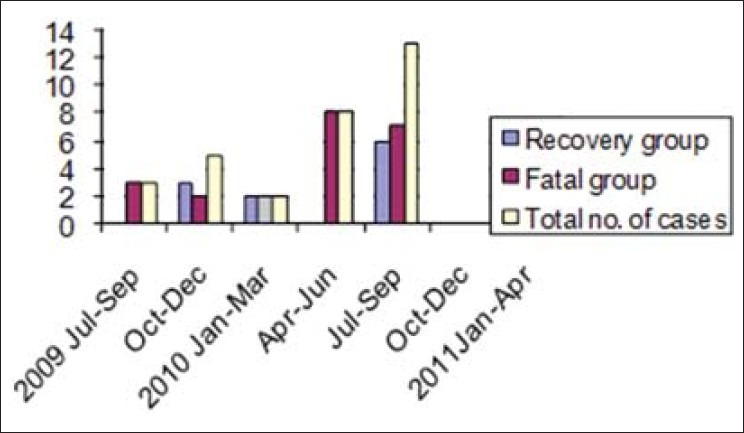
Bar diagram showing distribution of cases during months of epidemic
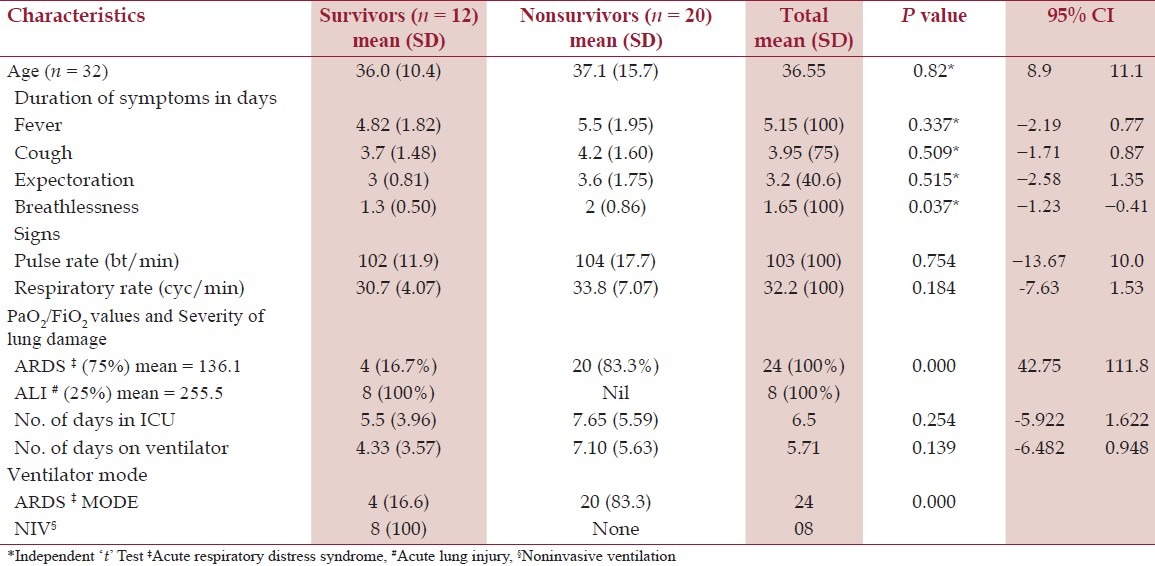
All the patients had tachycardia and tachypnea. Respiratory system examination showed bilateral/unilateral, diffuse/focal rhonchi and/ or crepitations. The mean of laboratory parameters such as hemoglobin (12 g/dl), total leukocyte count (7311 cells/mm3), and differential count (neutrophils – 72.7%, lymphocyte – 32%) was within the normal range. Serum creatinine was raised in eight cases; none progressed to acute kidney injury. Mild elevation of AST (Aspartate aminotransferase) and ALT ((Alanine aminotransferase) was seen in seven cases. The PCT was positive in 28.1% (9/32) and none of them were culture positive. Incidentally all PCT positive cases succumb to illness, with significant P value (P = 0.006). All the 32 cases showed varying degree lung infiltrations suggestive bronchopneumonia or ALI or ARDS on chest X-ray. The mean number of days in ICU was 5.5 and 7.6 days in survivors versus nonsurvivors, respectively. The mean number of days on ventilator support was 4.3 and 7.1 days in survivors versus nonsurvivors, respectively [Table 2]. The mean number of days in hospital was 8.5 and 7.7 days in survivors versus nonsurvivors, respectively. ARDS was seen in 75% (24/32) of study group, all were managed by invasive ventilation (IPPV/CMV) in ARDS mode (low tidal volume and high peep). Among ARDS cases four cases recovered. ALI was seen in eight cases, all are managed by noninvasive ventilatory support (CPAP/ BiPAP) and all cases recovered (P = 0.0001). To summarize, out of 32 patients with respiratory failure, 12 recovered and 20 succumbed to H1N1, all nonsurvivors had extensive lung damage in the form of ARDS (P = 0.0001).
Table 2.
Age, symptoms, signs, PaO2/FiO2 ratio, and patient care parameters among two groups of patients
Discussion
The emergent pandemic H1N1-2009 virus is challenging to the medical community around the world. Because of its unusual rapidity of spread specially among young and previously healthy patients, who were considered as low risk group for influenza-induced complications.[12–14] In the present study, majority (78.1%) of the cases were seen in age group of 15–45 years, was fatal in 60% (15/25). This is similar to study by Choudasama et al., in which mean age was 29.4 years[15] and by Chako et al. with cases in the age group of 19–55 years.[16] This may be due to immunity in older persons due to pre-2009 H1N1 influenza virus exposure. In future years, as the virus evolves, the epidemiology of H1N1-infection may affect older persons to a greater extent.[17–20]
In the present study, fatality was more in male (73.7%). This is in contrast to a recent Indian study where fatality was more in female, that is, 75%.[21] The chief presenting complaints were fever, breathless (100% each), and cough (75%); as comparable to studies by Rao et al. and Puvanalingam et al.[7,21] Underlying diseases/risk factor like diabetes mellitus, hypertension, ischemic heart disease, bronchial asthma, and pregnancy were seen in 46.8%, as comparable to 52.6% in Kerkhove et al. global pooled analysis of H1N1.[22] Among vital parameters all our patients had tachypnea and tachycardia similar to observation by Bewick et al. study.[23] The laboratory data such as complete blood count were within normal range, as comparable to other studies.[23] So this suggests that routine laboratory parameters did neither contribute to diagnosis nor management of H1N1 influenza.
In this study, PCT was positive in nine fatal cases, other studies also highlights importance of secondary bacterial pneumonias, such as staphylococcus aureus and streptococcus pneumonia.[5,24] But evidence of bacterial coinfection was seen in fewer than 30%, suggesting that primary viral pneumonia is often important.[25] All our cases were culture negative, this could be due to use of broad spectrum antibiotics. The chest X-ray of all (100%) the patients showed varying degree of lung infiltrations as comparable to study by Choudasama et al. with 93% showed lung infiltrations.[15] The mean of PaO2/FiO2 was 136 in ARDS group, is comparable to previous study, that is, 147.[5] ARDS secondary to H1N1-related viral pneumonia is the leading cause of morality in the present study and which is similar to previous studies.[5,24] ALI is a significant cause of morbidity and it can progress to ARDS.[5] Tablet oseltamivir was started immediately on an average 5 days postsymptomatic; this delay of 5 days is due to recognition of H1N1-infection by general practitioners. This could have contributed to rapid progression and development of complications in our cases. Previous studies have concluded early initiation of oral oseltamivir reduced morbidity and mortality.[11,26]
Limitations
This study is a retrospective observational study of patients treated in our ICU and findings have limited external validity in terms of generalizations, as it is a single centre study.
Conclusion
Fever and breathlessness were the main presenting complaints tachypnea and tachycardia, as clinical signs predict development of respiratory complications in H1N1 swine flu. ABG and PaO2/FiO2 are important in deciding severity of lung injury and mode of ventilation. ARDS was observed to be the main cause of mortality in the present study. Serum PCT level estimation is also useful in determining outcome.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Centers for Disease Control and Prevention (CDC). Outbreak of swine-origin influenza A (H1N1) virus infection- Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:467–70. [PubMed] [Google Scholar]
- 2.World Health Organization. World now at the start of 2009 influenza pandemic. In statement to the press by WHO director, 2009. (Accessed December 12, 2011, at http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_2009 061/en/index.html. )
- 3.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 4.Miller E, Hoschler K, Hardelid P, Stanford E, Andrewa N, Zambon M. Incidence of 2009 pandemic influenza A (H1N1) in England: A cross sectional serological study. Lancet. 2010;375:1100–8. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–9. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 6.Kotsimbos T, Waterer G, Jenkins C, Kelly PM, Cheng A, Hancox RJ. Influenza A /H1N1_09: Australia and New Zealand's winter of discontent. Am J Respir Crit Care Med. 2010;181:300–6. doi: 10.1164/rccm.200912-1878CP. [DOI] [PubMed] [Google Scholar]
- 7.Puvanalingam A, Rajendiran C, Sivasubramanian K, Ragunanthanan S, Suresh S, Gopalakrishnan S. Case Series Study of the Clinical Profile of H1N1 Swine Flu Influenza. J Assoc Physicians India. 2011;59:14–8. [PubMed] [Google Scholar]
- 8.Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121:258–64. doi: 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez-Cherit G, Lapinsky SE, Maciaas AE, Pinto R, Espisa-Perez L, de la Torre A, et al. Critically ill patients with 2009 Influenza A (H1N1) in Mexico. JAMA. 2009;302:1880–7. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 10.Bernard GR, Artigas A, Bregham KL, Carlet J, Falke K, Hudson L, et al. The American-European consensus conference on ARDS.Definitions, Mechanisms, relevant outcomes and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 11.WHO recommendations for Pharmacological management of Pandemic (H1N1) 2009 influenza and other influenza viruses. 2009. Aug 20, (Accessed December 25, 2011 at http://www.leb.euro.who.int/h1n1_guidelines_ pharmacological_mng.pdf. )
- 12.Chowell G, Bertozzi SM, Colchero MA, Lopez GH, Alpuche AC, Hernandez M, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361:674–9. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–9. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 14.Michaelis M, Doerr H, Cinatl JJ. Novel swine – origin influenza a virus in humans: Another pandemic knocking at door. Med Microbiol Immunol. 2009;1SSSS98:175–83. doi: 10.1007/s00430-009-0118-5. [DOI] [PubMed] [Google Scholar]
- 15.Choudasama RK, Patel UV, Verma PB. Hospitalizations associated with 2009 influenza A (H1N1) and seasonal influenza in Saurashtra region, India. J Infect Dev Ctries. 2010;23:834–41. doi: 10.3855/jidc.1049. [DOI] [PubMed] [Google Scholar]
- 16.Chacko J, Gagan B, Ashok E, Radha M, Hemanth HV. Critically ill patients with 2009 H1N1 infection in an Indian ICU. Indian J Crit Care Med. 2010;14:77–82. doi: 10.4103/0972-5229.68220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 18.Ross TM, Mohmood K, Crevar CJ, Schneider OK, Heaton PM, Bright RA. A trivalent virus like particle vaccine elicits protective immune responses againstmseasonal influenza strains in mice and ferrets. PLoS One. 2009;4:e6032. doi: 10.1371/journal.pone.0006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skountzou I, Koutsonanos DG, Kim JH, Powers R, Satyabhama L, Masseoud F, et al. Immunity to pre-1950 H1N1 influenza viruses confers cross-protection against the pandemic swine-origin 2009 A(H1N1) influenza virus. J Immunol. 2010;185:1642–9. doi: 10.4049/jimmunol.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenning LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, et al. Incidence and characteristics of viral community acquired pneumonia in adults. Thorax. 2008;63:42–8. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 21.Rao SR Jagannatha, Rao MJ, Swamy N, Umapathy BL. Profile of H1N1 infection in tertiary care center. Indian J Pathol Microbiol. 2011;54:323–5. doi: 10.4103/0377-4929.81618. [DOI] [PubMed] [Google Scholar]
- 22.Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo GG, Koukounari A, Donnelly CA, et al. Risk Factors for Severe Outcomes following 2009 Influenza A (H1N1) Infection: A Global Pooled Analysis. PLoS Med. 2011;8:e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bewick T, Myles P, Greenwood S, Nguyen-Van-Tam JS, Brett SJ, Semple MJ, et al. Clinical and laboratory features distinguishing pandemic H1N1 influenza – related pneumonia from interpandemic community acquired pneumonia in adults. Thorax. 2011;66:247–52. doi: 10.1136/thx.2010.151522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. Factors associated with death or hospitalisation due to pandemic 2009 influenza. JAMA. 2009;302:1896–902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC). Bacterial coinfections in lung tissue specimens from fatal cases of swine-origin pandemic influenza A (H1N1) May-August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–4. [PubMed] [Google Scholar]
- 26.Sertogullarindan B, Ozbay B, Gunini H, Sunnetcioglu A, Arisoy A, Bilgin HM, et al. Clinical and prognostic features of patients with pandemic 2009 influenza A (H1N1) virus in intensive care unit. Afr Health Sci. 2011;11:163–70. [PMC free article] [PubMed] [Google Scholar]


