Sir,
Prostate cancer (PC) is the most common malignant tumor in men after lung neoplasm.[1,2] Positron emission tomography (PET) combined with computed tomography (PET/CT), using 18F- or 11C-Choline as radiotracer, provides good results in the re-staging of PC patients after prostatectomy.[3–10] This molecule, being a normal constituent of membrane cells phospholipids, represents a cell proliferation marker and is characterized by an intense accumulation in PC cells.
The aim of this study was to try to optimize 18F-Choline PET/CT acquisition protocol in PC patients with biochemical relapse after radical prostatectomy, by means of a blinded image analysis, in order to obtain all the useful information for re-staging, and lowering the absorbed dose as low as possible.
We started with an acquisition protocol including five PET/CT scans and applying it to 30 PC patients with disease relapse after radical prostatectomy: (i) early dynamic scan of the pelvis, (ii) early static scan of the pelvis, (iii) early whole-body scan, (iv) delayed static scan of the pelvis, and (v) delayed whole-body scan.
The blinded semi-quantitative image analysis showed that scan (v) provided the best information about local recurrences and distant metastases, while scan (ii) allowed to distinguish foci of radioactive urine in the bladder and urethra from loco-regional recurrences. The (i), (iii), and (iv) scans did not provide further useful information.
The PET/CT scanner installed in our centre is a Discovery STE hybrid scanner (General Electric, Milwaukee, USA). The maximum field-of-view (FOV) has a diameter of 70 cm and an axial extension (AFOV) of 15.7 cm, acquiring 47 slices of 3.3 mm thickness. The 16-slices integrated CT scanner Lightspeed 16 (General Electric) is equipped with a current modulation algorithm (smart-mA) to reduce radiation exposure.
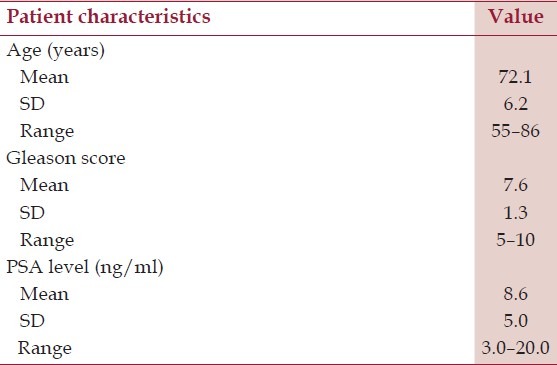
Up to now 356 patients underwent 18F-choline PET/CT investigation in our department. All these patients, already radically treated for PC, underwent a PET/CT exam for re-staging purpose. In order to choose the most appropriate acquisition procedure, we initially tested a ‘Five-Step protocol’ on 30 patients. These patients presented with biochemical recurrence (prostate-specific antigen [PSA] > 0.2 ng/mL) and negative or inconclusive conventional imaging (contrast-enhanced CT of pelvis and/or transrectal ultrasound [TRUS]). Patients’ characteristics are summarized in Table 1.
Table 1.
Patients clinical, biochemical, and histological characteristics
Patient preparation instructions before 18F-Choline PET/CT were: (1) fasting condition for at least 6 hours; and (2) no liquid ingestion from at least 1 hour before the examination. The acquisition procedure included five PET/CT scans (dynamic and static) acquired at different time intervals from injection, in order to obtain a complete set of information about 18F-Choline biodistribution. This allowed to follow 18F-Choline urinary excretion, according to previous works.[9,10] Based on subsequent analysis performed by three experienced nuclear medicine physicians by consensus, the acquisition sequence was optimized.
The first protocol adopted included five PET/CT scans, starting immediately after the intravenous injection of 3.0 MBq/kg body weight of 18F-Choline (IASOCholine, ARGOS Zyklotron GesmbH Linz, Austria):
First step: dynamic 18F-Choline PET/CT of the prostatic region:
Low-dose helical CT acquisition of the pelvis.
Dynamic 3D PET acquisition: 1 PET bed position, 10 min AFOV duration.
Second step: early static 18F-Choline PET/CT of the prostatic region soon after urination (within 12 min from injection):
Low-dose helical CT acquisition of the pelvis.
Static 3D PET acquisition: 1 PET bed position, 4 min AFOV duration.
Third step: early whole-body 18F-Choline PET/CT (20 min postinjection):
Low-dose whole-body helical CT acquisition, feet–head direction.
Whole-body 3D PET acquisition: slice overlap 7, 3 min AFOV duration, feet–head direction.
Fourth step: delayed static 18F-Choline PET/CT of the prostatic region (55 min after injection): with the same procedure as second step.
Fifth step: delayed whole-body 18F-Choline PET/CT (1 hour postinjection): with the same parameters and procedure as third step.
Each patient was positioned supine on the scanner bed with the arms crossed above the head, localizing the laser alignment system on the iliac spine. Each CT acquisition was preceded by a scout-view (80 kV, 10 mA, antero-posterior) to set PET/CT scan limits: in the pelvis acquisitions (first, second, and fourth step), the scan superior border coincided with the upper portion of the iliac bone and the pubic symphysis had to stay on the scan centre, in order to include prostate and regional lymph node, comprising the inguinal ones. For the whole-body acquisitions (third and fifth step), the scan ranged from the orbito-meatal level to the superior portion of the thigh. The low-dose CT scans were used for attenuation correction in the subsequent PET acquisition. Two types of CT scans were acquired: the helical CT scan of the pelvis (120 kV, 80 mA, slice thickness 3.75 mm, pitch 1.375, speed 13.75 mm/rot, rotation time 0.8 s, 50 cm FOV diameter) and the whole-body helical CT scan (120 kV, 40–100 mA modulated by GE Smart-mA with a noise index of 30, slice thickness 3.75 mm, pitch 1.375, speed 13.75 mm/rotation, rotation time 0.8 s, 50 cm FOV diameter). All PET scans were acquired in 3D mode, with FOV diameter 50 cm; data were collected in list mode and reconstructed by the VUE- Point (General Electric, Milwaukee, USA) fully-3D iterative reconstruction algorithm, with 20 subsets by 2 iterations, 128 × 128 matrix.
The image analysis was performed in a XELERIS workstation (version 2.1753, General Electric, Milwaukee, USA), using a dedicated protocol to review the fused PET/CT images, also quantitatively, in terms of standardized uptake value (SUV) calculated according to the equation:
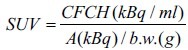
where CFCH is the concentration of 18F-Choline activity in the attenuation-corrected PET images, b.w. is the body weight of the patient, and A is the 18F-Choline activity at the start of PET acquisition; the ratio A/b.w. was equal to 3.0 kBq/g. The conversion from counts to CFCH (kBq/ml) is performed by the GE image elaboration software using the calibration data.
Every focal tracer accumulation deviating from the physiological distribution was regarded as being positive, in particular if accumulated in the prostatic fossa, in lymph nodes and in the skeletal system. Also the tumor-to-background ratio (TBR) was computed, calculating the ratio between the SUVmax value in the lesion and the mean SUV in a background region-of-interest (ROI), placed in the contralateral normal soft tissue, approximately matching the diameter of the tumor.
Histological findings obtained by biopsy or surgery were taken as gold standard.
For patient dosimetry, we calculated the effective dose of each CT scan for a standard man, by means of the software CT Expo (version 2.0.1, Medizinische Hochschule, Germany),[11] while the 18F-Choline PET contribution was calculated according to data published by De Grado et al.[12,13] The CT Expo software uses the technical scan parameters, together with Monte Carlo simulations, to estimate the volumetric computed-tomography dose index (CTDIvol), the absorbed dose to the organs of a standard patient and finally the effective dose (E) to the standard patient. We performed calculations according only to the more recent ICRP 103 recommendations.[14]
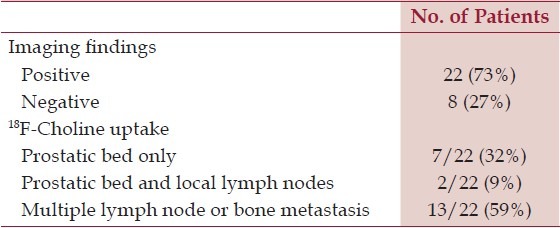
The blinded imaging analysis of the 150 PET/CT scans acquired according to the ‘Five-Step Protocol’ was conducted by three experienced nuclear medicine physicians and results are summarized in Table 2. All the 22 18F-Choline positive patients were true positive according to histological findings. No false positive cases were observed in our series.
Table 2.
The PET/CT detection rate and 18F-Choline uptake obtained for the re-staging of 30 patients with biochemical recurrence after radical prostatectomy
The 1-hour delayed whole-body 18F-Choline PET/ CT (fifth step of the protocol) provided the best information about the prostate bed relapse, the local node recurrences, and distant metastases. In this regard it is important to point out that both SUVmax and TBR values significantly increased in delayed 1-hour scans, as shown in Table 3. Furthermore, the early static 18F-Choline PET/CT (second step) of the prostatic region was very important to identify the physiologic radioactive excretion through the prostatic urethra and bladder, therefore allowing to distinguish radioactive urine from loco-regional recurrences [Figures 1 and 2]. In fact, in three patients out of the nine locally recurrent patients (33%), the prostatic bed uptake was detected in the early static scan while it was obscured by urinary radioactivity on delayed imaging.
Table 3.
SUVmax and TBR mean values (and range) obtained in the second, third, and fifth step 18F-Choline PET/CT scans for the 37 Choline-positive lesions
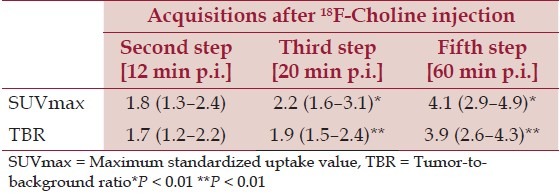
Figure 1.
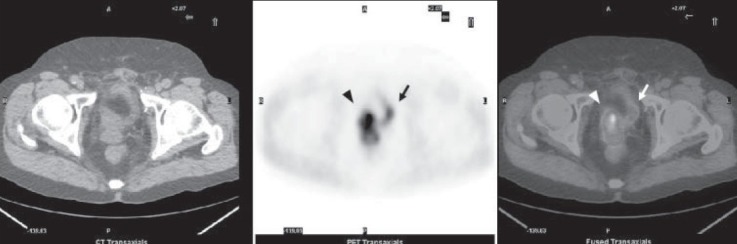
5-min early acquisition imaging. From left to right: low-dose CT, PET, fused PET/CT axial images of the pelvis showing a prostate cancer relapse in the right side of prostate bed (arrow head) while bladder is substantially empty (arrow)
Figure 2.
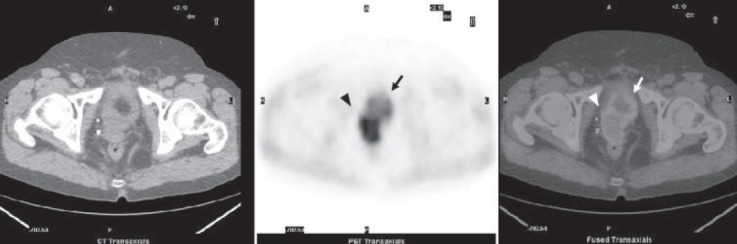
60-min delayed acquisition imaging. From left to right: low-dose CT, PET, fused PET/CT axial images of the pelvis showing a prostate cancer relapse in the right side of prostate bed (arrow head) and a diffuse uptake in the bladder (arrow)
Conversely, in our experience, the early dynamic, early whole-body and delayed static PET/CT scans (step first, third, and fourth) did not provide further relevant information to change the exam interpretation.
Regarding the patients’ dosimetry, the effective dose due to CT scans and 18F-Choline kinetics are shown in Table 4. For the whole-body scan with automatic mA modulation, the current was set to 90 mA, in order to obtain CTDIvol and DLP values in good agreement with the console (within 5%), values verified also by means of phantom measurements.
Table 4.
The Effective dose and CTDIvol values calculated by means of the software CT Expo.[13] The effective dose due to the five CT scans and the 18F-Choline uptake is reported below

After radical treatment of PC, PSA level decreases until becoming undetectable. If PSA levels rise (≥0.2 ng/ mL, or progressive increment of PSA level), recurrence must be suspected.[15,16] The identification of the site of the recurrence is very important to choose the most suitable therapeutic procedure. In this respect, the use of radio-Choline PET/CT imaging has proved favorable results with high sensitivity in detecting PC tumor recurrences.[3–10] The main characteristics of Choline are: (a) urinary excretion begins 3–5 min after injection; (b) rapid uptake by PC cells with a maximum peak at 55 min postinjection; and (c) rapid blood clearance.
Choline can be labeled with both the positron emitters 11C or 18F. 11C-Choline shows low renal excretion, resulting in accurate detection of recurrences in the prostatic region and loco-regional lymph nodes.[5–7] However, 11C-Choline has a short half-life (20 min) requiring a short time (minutes) between synthesis and PET acquisition. Conversely, 18F-Choline is characterized by a longer half-life (110 min) that allows delayed acquisition, until 1 hour or more postinjection, when the maximum uptake by PC is reached:[7,8] this phenomenon has been confirmed also by our data about SUVmax and TBR increase during time [Table 3]. Alternatively, a potential drawback of 18F-Choline is its variable urinary excretion with high accumulation in the bladder that can compromise a correct evaluation of the prostatic region. Most of the studies reported in literature have been performed with 11C-Choline to detect metastatic deposits in cases of biochemical relapse.[17] Instead a few data have been reported with 18F-Choline and there is still a debate about the best acquisition protocol to be adopted.
According to our preliminary data about 18F-Choline kinetics and biodistribution observed in a group of 30 patients using a five-step PET/CT protocol, the early static 18F-Choline PET/CT scan of the pelvis combined with the delayed 1-hour whole-body scan were able to provide the greatest amount of information for the re-staging purpose. Unlike other data reported in literature,[9,10] the early dynamic and the delayed static scans of the prostatic region did not give further useful information.
An early whole-body scan acquired a few minutes after radiotracer injection is mandatory when Choline is labeled with 11C because of its short half-life; on the contrary, using 18F-Choline, as in our study, delayed scans can be acquired, providing better quality imaging (with significantly higher SUVmax and TBR values in comparison with early images, Table 3), therefore allowing more confidence in reading and interpreting scans by nuclear medicine physician.
It is worth noting that the effective dose of a 18F-Choline PET/CT examination using the ‘Five-Step protocol’ was 23.4 mSv (calculated for a 70 kg standard man), with 16.9 mSv ascribable to CT examinations, which is similar to that of a routine contrast-enhanced CT exam of the abdomino-pelvic region (about 20 mSv).[18] Moreover, the ‘Five-Step protocol’ we initially used was time-consuming. On the basis of the above considerations, we modified our routine 18F-Choline PET/CT protocol into the ‘two-step’ one:
First step: early static 18F-Choline PET/CT of the pelvis (within 3–5 min from injection, soon after bladder void):
Low-dose helical CT acquisition of the pelvis.
Static 3D PET acquisition: 1 PET bed position, 4 min AFOV duration.
Second step: delayed 1-hour whole-body 18F-Choline PET/CT (55–65 min postinjection):
Low-dose whole-body helical CT acquisition, feet-head direction.
Whole-body 3D PET acquisition: slice overlap 7, AFOV duration 3 min, feet–head direction.
The patient positioning and the settings used in CT and PET scans were the same as the ‘five-step protocol’. The first early static pelvic acquisition allowed to study prostate region before physiological urinary excretion, while the delayed whole-body scan was useful to confirm the findings in the pelvis and to evaluate the presence of distant metastases. Acquiring the early static PET/ CT scan at 3–5 min from injection reduced interference from urinary excretion, which starts about 5 min after injection. In addition, the standard patient's effective dose was reduced to 14.5 mSv by reducing the number of CT scans performed.
An interesting observation in our series lies in the low fraction of patients with local relapse alone (32%), therefore suitable for a therapeutic approach with radical purpose, while in 59% of cases distant relapses were present (with or without local relapse, Table 2), often with bone spread, thus treated with systemic anti-androgenic treatment,
The 18F-Choline PET/CT examination showed a relevant impact on the patient management, allowing the distinction between patients treatable with local radiation therapy,[19] from those with distant spread in whom systemic therapy was planned.
In conclusion, on the basis of the data of the present study, a ‘two-step’ 18F-Choline PET/CT protocol based on early (3–5 min) and delayed (1 hour) acquisition seems adequate enough to obtain good quality imaging to visualize recurrent disease and at the same time to avoid false-positive results related to urinary excretion.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Siegel R, Bray F, Forman D, Mathers C, Parkin DM. Lyon: International Agency for Research on Cancer; 2010. [Last accessed on 2011 Nov 25]. GLOBOCAN 2008 Cancer incidence and mortality worldwide: IARC cancerbase No.10. Available from: http://globocan.iarc.fr/ [Google Scholar]
- 3.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F or 11C-Acetate and 18F- or 11C-Choline. J Nucl Med. 2011;52:81–9. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reske SN, Blumstein NM, Glatting G. [11C]Choline PET/CT imaging in occult local relapse of prostate cancer after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2008;35:9–17. doi: 10.1007/s00259-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 5.Farsad M, Schiavina R, Castellucci P, Nanni C, Corti B, Martorana G, et al. Detection and localization of prostate cancer: Correlation of 11C-Choline PET/CT with histopathologic step-section analysis. J Nucl Med. 2005;46:1642–9. [PubMed] [Google Scholar]
- 6.Castellucci P, Fuccio C, Marzola MC, Al-Nahhas A, Rubello D, Fanti S. Prostate-specific antigen kinetics and choline PET/CT in patients with biochemical relapse after primary treatment for prostate cancer. Nucl Med Commun. 2011;32:475–8. doi: 10.1097/MNM.0b013e3283455765. [DOI] [PubMed] [Google Scholar]
- 7.DeGrado TR, Coleman RE, Wang S, Baldwin SW, Orr MD, Robertson CN, et al. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: Initial findings in prostate cancer. Cancer Res. 2000;61:110–7. [PubMed] [Google Scholar]
- 8.Husarik DB, Miralbell R, Dubs M, John H, Giger OT, Gelet A, et al. Evaluation of [18F]-choline PET/CT for staging and restaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:253–63. doi: 10.1007/s00259-007-0552-9. [DOI] [PubMed] [Google Scholar]
- 9.Heinisch M, Dirisamer A, Loidl W, Stroiber F, Gruy B, Haim S, et al. Positron emission tomography/computed tomography with F-18-fluorocholine for restaging of prostate cancer patients: meaningful at PSA < 5 ng/ml? Mol Imaging Biol. 2006;8:43–8. doi: 10.1007/s11307-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 10.Hacker A, Jeschke S, Leeb K, Prammer K, Ziegerhofer J, Sega W, et al. Detection of pelvic lymph node metastases in patients with clinically localized prostate cancer: Comparison of [18F]Fluorocholine positron emission tomography-computerized tomography and laparoscopic radioisotope guided sentinel lymph node dissection. J Urol. 2006;176:2014–9. doi: 10.1016/j.juro.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Stamm G, Nagel HD. CT-Expo - a novel program for dose evaluation in CT. Rofo. 2002;74:1570–6. doi: 10.1055/s-2002-35937. [DOI] [PubMed] [Google Scholar]
- 12.DeGrado TR, Reiman RE, Price DT, Wang S, Coleman RE. Pharmacokinetics and radiation dosimetry of 18F-fluorocholine. J Nucl Med. 2002;43:92–6. [PubMed] [Google Scholar]
- 13.Erratum in: DeGrado TR, Reiman RE, Price DT, Wang S, Coleman RE. J Nucl Med. 2002;43:509. [PubMed] [Google Scholar]
- 14.Vol. 37. ICRP Publication 103. Ann. ICRP; 2008. ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. [DOI] [PubMed] [Google Scholar]
- 15.Freedland SJ, Presti JC, Amling CL, Kane CJ, Aronson WJ, Dorey F, et al. Time trends in biochemical recurrence after radical prostatectomy: Results of the search database. Urol. 2003;61:736–41. doi: 10.1016/s0090-4295(02)02526-8. [DOI] [PubMed] [Google Scholar]
- 16.Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–23. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 17.Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, et al. Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010;37:301–9. doi: 10.1007/s00259-009-1253-3. [DOI] [PubMed] [Google Scholar]
- 18.Vol. 37. Stockholm, Sweden: ICRP Publication 102. Ann. ICRP; 2007. ICRP. Managing patient dose in Multi-Detector Computed Tomography (MDCT) [DOI] [PubMed] [Google Scholar]
- 19.Würschmidt F, Petersen C, Wahl A, Dahle J, Kretschmer M. [18F]fluoroethylcholine-PET/CT imaging for radiation treatment planning of recurrent and primary prostate cancer with dose escalation to PET/CT-positive lymph nodes. Radiat Oncol. 2011;6:44. doi: 10.1186/1748-717X-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]


