Abstract
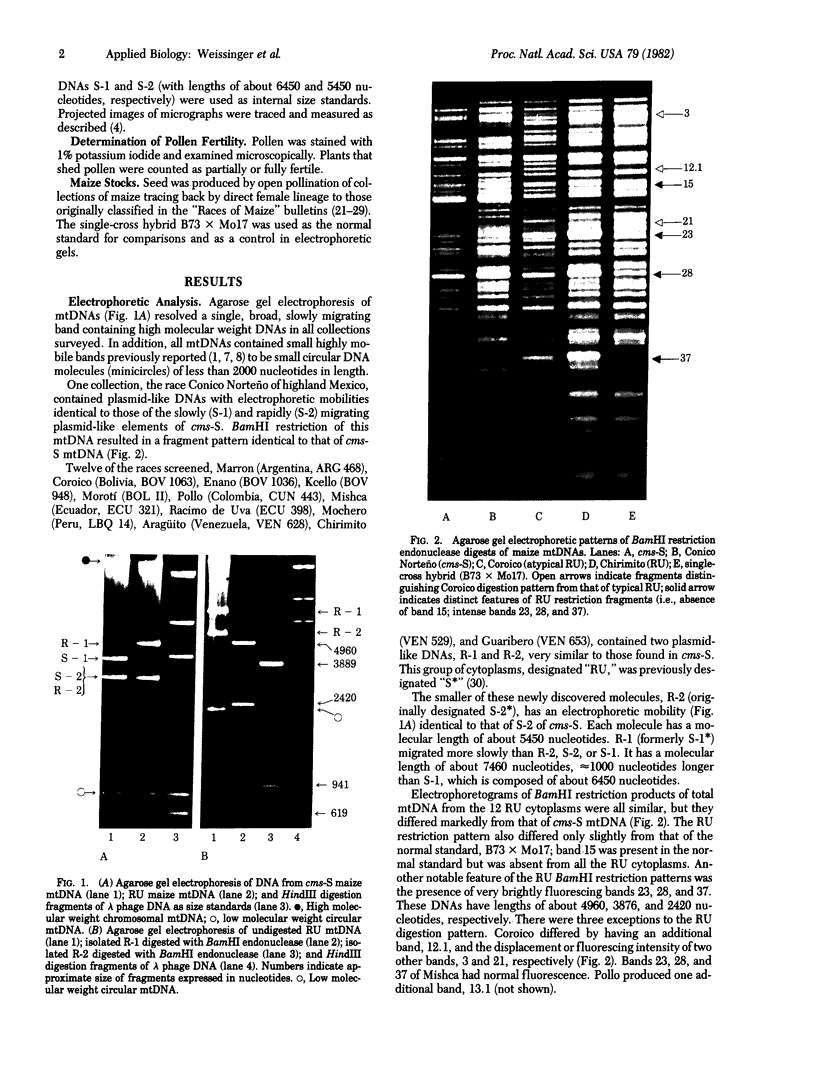
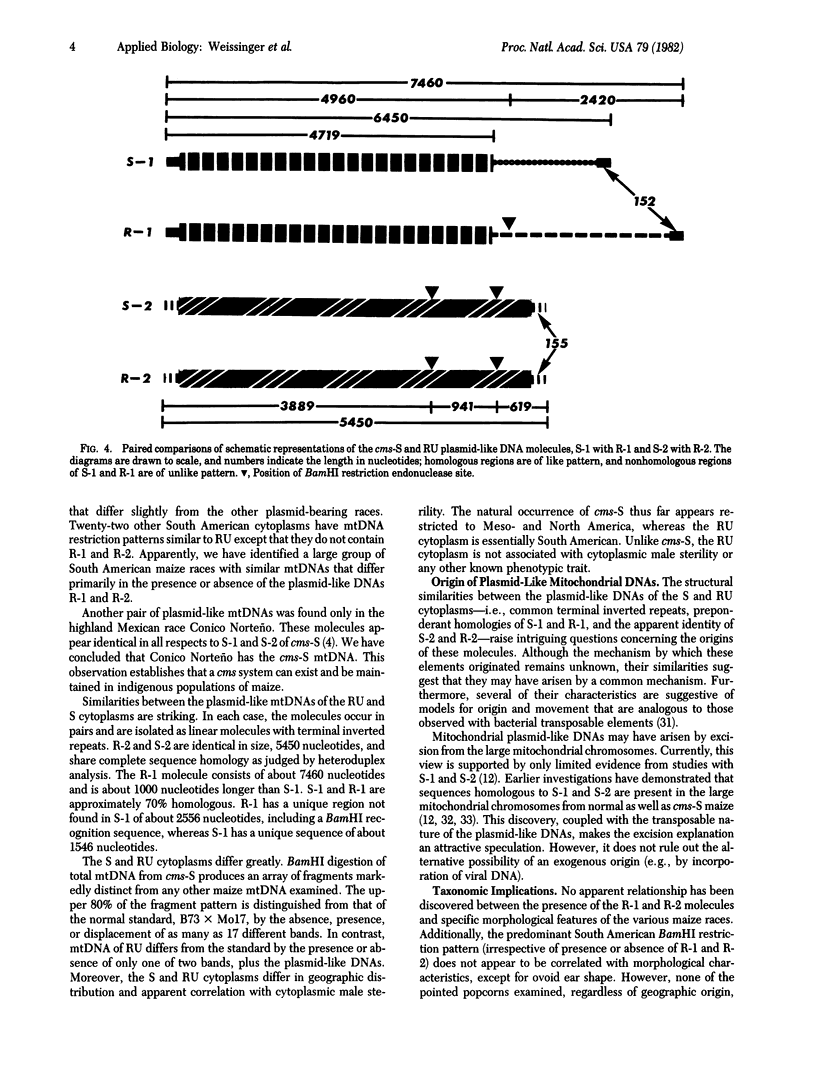
Mitochondrial DNA from 81 races of Latin American maize were examined by agarose gel electrophoresis. Twelve South American races each contained two plasmid-like mtDNA molecules similar to those of the cytoplasmic male-sterile S type (cms-S). The plasmid-like elements from all 12 races, designated RU, appear to be identical. Both molecules appear in vitro as double-stranded linear DNAs terminated by repeated sequences arranged in reverse polarity (terminal inverted repeats). The larger molecule of the pair, R-1, contains about 7460 nucleotides. It shares considerable homology with the larger plasmid-like molecule of cms-S, S-1, but is about 1000 nucleotides longer than S-1, has a unique sequence of about 2576 nucleotides, and also contains a BamHI recognition site not present in S-1, R-2, the smaller plasmid-like element, consists of about 5450 nucleotides and appears to share complete homology with S-2, the smaller plasmid-like molecule of cms-S. Neither pollen sterility nor any other trait has been associated with the R-1 and R-2 plasmid-like mtDNAs. The BamHI restriction fragments of total mtDNA from the 12 RU cytoplasms display similar patterns, which differ only slightly but vividly from that of a normal maize standard, B73 × Mo17. BamHI restriction analysis of 22 additional races produced arrays similar to those of the RU cytoplasms, but which lacked plasmid-like mtDNAs. The taxonomic significance of this digestion pattern and of the RU cytoplasms is discussed. One Mexican race, Conico Norteño, has been shown to contain the cms-S cytoplasm.
Keywords: maize systematics, restriction endonuclease fragment analysis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Kahmann R., Kamp D. Electron microscopic characterization of DNAs of non-defective deletion mutants of bacteriophage Mu. J Mol Biol. 1977 Jul 15;113(4):591–609. doi: 10.1016/0022-2836(77)90224-8. [DOI] [PubMed] [Google Scholar]
- Cohen S. N. Transposable genetic elements and plasmid evolution. Nature. 1976 Oct 28;263(5580):731–738. doi: 10.1038/263731a0. [DOI] [PubMed] [Google Scholar]
- Kemble R. J., Gunn R. E., Flavell R. B. Classification of Normal and Male-Sterile Cytoplasms in Maize. II. Electrophoretic Analysis of DNA Species in Mitochondria. Genetics. 1980 Jun;95(2):451–458. doi: 10.1093/genetics/95.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Inverted repeats in chloroplast DNA from higher plants. Proc Natl Acad Sci U S A. 1979 Jan;76(1):41–45. doi: 10.1073/pnas.76.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Kim B. D., Pring D. R., Conde M. F., Mans R. J., Laughnan J. R., Gabay-Laughnan S. J. Cytoplasmic Reversion of cms-S in Maize: Association with a Transpositional Event. Science. 1980 Aug 29;209(4460):1021–1023. doi: 10.1126/science.209.4460.1021. [DOI] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Pring D. R. Restriction endonuclease analysis of mitochondrial DNA from normal and Texas cytoplasmic male-sterile maize. Science. 1976 Jul 9;193(4248):158–160. doi: 10.1126/science.193.4248.158. [DOI] [PubMed] [Google Scholar]
- Pring D. R., Levings C. S. Heterogeneity of Maize Cytoplasmic Genomes among Male-Sterile Cytoplasms. Genetics. 1978 May;89(1):121–136. doi: 10.1093/genetics/89.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring D. R., Levings C. S., Hu W. W., Timothy D. H. Unique DNA associated with mitochondria in the "S"-type cytoplasm of male-sterile maize. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2904–2908. doi: 10.1073/pnas.74.7.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer H. E., Sederoff R. R. Improved estimation of DNA fragment lengths from Agarose gels. Anal Biochem. 1981 Jul 15;115(1):113–122. doi: 10.1016/0003-2697(81)90533-9. [DOI] [PubMed] [Google Scholar]
- Smith H. O. Recovery of DNA from gels. Methods Enzymol. 1980;65(1):371–380. doi: 10.1016/s0076-6879(80)65048-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Measurement of DNA length by gel electrophoresis. Anal Biochem. 1979 Dec;100(2):319–323. doi: 10.1016/0003-2697(79)90235-5. [DOI] [PubMed] [Google Scholar]
- Thompson R. D., Kemble R. J., Flavell R. B. Variations in mitochondrial DNA organisation between normal and male-sterile cytoplasms of maize. Nucleic Acids Res. 1980 May 10;8(9):1999–2008. doi: 10.1093/nar/8.9.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Timothy D. H., Levings C. S., Pring D. R., Conde M. F., Kermicle J. L. Organelle DNA variation and systematic relationships in the genus Zea: Teosinte. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4220–4224. doi: 10.1073/pnas.76.9.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]