Abstract
In this paper we extend and reconsider a solitonic model of the actionpotential in biological membranes for the case of plant cells. Aiming togive at least a qualitative description of the K+,Cl- and Ca2+ driven process of propagation ofthe action potential along plant cells we put forward the hypothesis ofthree scalar fields φi (X), i = 1, 2, 3 which representK+, Cl- and Ca2+ ions,respectively. The modulus squared of these fields carries the usualquantum-mechanical (probabilistic) interpretation of the wave function. Onthe other hand, the fields are described themselves by the Lagrangiandensities ℒ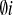 . Moreover, the interaction and self-interaction term ℒ
. Moreover, the interaction and self-interaction term ℒ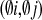 between thefields is considered. The Lagrangian densities ℒ
between thefields is considered. The Lagrangian densities ℒ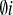 include a double-well potential (which is proportional toσ4i) that leads to spontaneous symmetrybreaking which may produce structures with non-zero topological charge, e.g.longitudinal solitons. In order to describe the transversal motion of theions of concern we need to assume only non-uniform solutions of the system of equation of motion. Hence we seek for solutions (travelling waves) whichpreserve the shape and which move without dissipation and in this way wereconstruct the main dynamical features of the action potential in plants.
include a double-well potential (which is proportional toσ4i) that leads to spontaneous symmetrybreaking which may produce structures with non-zero topological charge, e.g.longitudinal solitons. In order to describe the transversal motion of theions of concern we need to assume only non-uniform solutions of the system of equation of motion. Hence we seek for solutions (travelling waves) whichpreserve the shape and which move without dissipation and in this way wereconstruct the main dynamical features of the action potential in plants.
Keywords: Action potentials, Plants, Ion fluxes, Longitudinal solitons
Full Text
The Full Text of this article is available as a PDF (510.7 KB).
References
- Abe S., Takeda J., Senda M. Restingmembrane potential and action potential of Nitella expansa protoplasts. Plant and Cell Physiol. 1980;21:537–546. [Google Scholar]
- Abe T. Chloride ion efflux during an action potential in the main pulvinus of Mimosa pudica. Bot. Magaz.(Tokyo) 1981;94:379–383. [Google Scholar]
- Barrett T. W. Energy transfer and molecular switching. I. The nerve action potential. J. Theoret. Biol. 1981;92:185–207. doi: 10.1016/0022-5193(81)90287-3. [DOI] [PubMed] [Google Scholar]
- Beilby M. J., Coster H. G. L. The action potential in Chara corallinaII. Two activation-inactivation transients in voltage clamps of plasmalemma. Austalian J. Plant Physiol. 1979;6:329–335. [Google Scholar]
- Beilby M.J. Calcium and Plant Action Potentials. Plant, Cell and Environ. 1984;7:415–421. [Google Scholar]
- Coleman H. A. Chloride currents in Chara–A patchclamp study. J.Membr. Biol. 1986;93:55–61. [Google Scholar]
- Davies E., Schuster A. Wounding, action potentials and polysome formation. Plant Physiol. 1981;67:538. [Google Scholar]
- Davies E., Schuster A. Intercellular communication in plants: Evidence for a rapidly generated, bidirectionally transmitted wound signal. Proceedings of the National Academy of Sciences, U.S.A. 1981;78:2422–2426. doi: 10.1073/pnas.78.4.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E. Action potentials as multifunctional signals in plants: a unifying hypothesis to explain apparently disparate wound responses. Plant, Cell and Environ. 1987;10:623–631. [Google Scholar]
- Filek M., Pazurkiewicz-Kocot K., Dubert F., Marcińska I., Biesaga-Kościelniak J. Changes of surface potential and phospholipid composition of winter wheat callus cells grown at 5 °C and 25 °C. J. Agron. Crop Sci. 1993;171:243–250. [Google Scholar]
- Fromm J., Eschrich W. Electric signals released fromroots of willow (Salix viminalisL.) change photosynthesis and transpiration. J. Plant Physiol. 1993;141:673–680. [Google Scholar]
- Fromm J., Spanswick R. Characteristics of action potentials in willow (Salix viminalisL.) J. Exp. Bot. 1993;44:1119–1125. [Google Scholar]
- Fromm J., Bauer T. Action potentials in maize sieve tubes change phloem translocation. J. Exp. Bot. 1994;45:463–469. [Google Scholar]
- Gaffey C. T., Mullins L. J. Ion fluxes during the action potential in Chara. J. Physiol. 1958;144:505–524. doi: 10.1113/jphysiol.1958.sp006116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy A. The evolution of plant action potentials. J. Theoret. Biol. 1983;103:645–648. [Google Scholar]
- Gradmann D. ‘Metabolic’ action potentials in Acetabularia. J. Membr. Biol. 1976;29:23–45. doi: 10.1007/BF01868950. [DOI] [PubMed] [Google Scholar]
- Gradmann D., Mummert H. ‘Plant action potentials’. In: Spanswick R. M., Lucas W. J., Dainty J., editors. Plant Membrane Transport: Current Conceptual Issues. Amsterdam: Elsevier/NorthHolland Biomedical Press; 1980. pp. 333–347. [Google Scholar]
- Hodgkin A. L., Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A.L., Huxley A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Huxley A. F., Katz B. Measurements of current-voltage relations in the membrane of the giant axon of Loligo. J. Physiol. 1952;116:424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope A.B., Walker N.A. The Physiology of Giant Algal Cells. Cambridge: Cambridge University Press; 1975. Action potentials in Charophytecells; pp. 111–119. [Google Scholar]
- Keynes R. D. Ion channels in the nerve-cell membrane. Sci. American. 1979;240:126–135. doi: 10.1038/scientificamerican0379-126. [DOI] [PubMed] [Google Scholar]
- Kikuyama M., Tazawa M. Transient increase of intracellular Ca2+ during excitation of tonoplast-free Characells. Protoplasma. 1983;117:62–67. [Google Scholar]
- Kikuyama Ion efflux during a single action potential of Nitella axilliformisin medium lacking Ca2+ Plant and Cell Physiol. 1987;28:179–186. [Google Scholar]
- Lakshminarayanaiah N. Equations of Membrane Biophysics. Orlando, San Diego: Academic Press; 1984. [Google Scholar]
- Lunevsky V.Z., Zherelova O.M., Vostrikov I.Y., Berestovsky G.N. Excitation of Characeae cell membranes as a result of activation of calcium and chloride channels. J. Membr. Biol. 1983;72:43–58. [Google Scholar]
- Mańka R., Ogrodnik B. Soliton model of transport along microtubules. J. Biol. Phys. 1991;18:185–189. [Google Scholar]
- Maśka M., Pietruszka M. On the ø4 field theoretical model for the action potential. Journ. of Biol. Phys. 1995;21:211–222. [Google Scholar]
- Nossal, R. and Lecar, H.: Molecular and Cell Biophysics(1991).
- Odell G. M. ‘Biological waves’. In: Segel L.A., editor. Mathematical Models in Molecular and Cellular Biology. Cambridge: Cambridge University Press; 1980. [Google Scholar]
- Pickard B. G. Action potentials in higher plants. The Bot. Rev. 1973;39:172–201. [Google Scholar]
- Pietruszka M. Time evolution of the action potential by the fourth-order Runge-Kutta method. Current Topics in Biophys. 1993;17:35–39. [Google Scholar]
- Roblin G. Analysis of the variation potential induced by wounding in plants. Plant and Cell Physiol. 1985;26:455–461. [Google Scholar]
- Russell, J. Scott: Brit. Assoc. Rep.(1844).
- Samejima M., Sibaoka T. Changes in the extracellular ion concentration in the main pulvinus of Mimosa pudicaduring rapid movement and recovery. Plant and Cell Physiol. 1980;21:467–479. [Google Scholar]
- Shiina T., Tazawa M. Action potential in Luffa cylindricaand its effects on elongation growth. Plant and Cell Physiol. 1986;27:1081–1089. [Google Scholar]
- Shiina T., Tazawa M. Ca2+-activated Cl- channel in plasmalemma of Niteloptis obtusa. J. Membr. Biol. 1987;53:137–146. [Google Scholar]
- Shimmen T., Tazawa M. Intracellular chloride and potassium ions in relation to excitability of Charamembrane. J. Membr. Biol. 1980;55:223–232. [Google Scholar]
- Simons P. J. The role of electricity in plant movements. New Phytologist. 1981;87:11–37. [Google Scholar]
- Slayman C. L., Long W. S., Gradmann D. Action potentials in Neurospora crassaa mycelial fungus. Biochimica et Biophysica Acta. 1976;426:732–744. doi: 10.1016/0005-2736(76)90138-3. [DOI] [PubMed] [Google Scholar]
- Stolarek J., Pazurkiewicz-Kocot K. Light induced action potentials in Phaseolus vulgarisL. Acta Biol. Silesiana. 1980;9:9–17. [Google Scholar]
- Stolarek J., Pazurkiewicz-Kocot K., Zientara M. The action of phytohormones on resting and action potential in higher plants. Post. Biol. Kom. 1984;11:361–363. [Google Scholar]
- Trębacz K. Light-triggered action potentials in plants. Acta Soc. Bot. Poloniae. 1989;58:141–156. [Google Scholar]
- Tsutsui I., Ohkawa T., Nagai R., Kishimoto U. Inhibition of Cl- channel activation in Chara corallinamembrane by lanthanum ion. Plant and Cell Physiol. 1986;27:1197–1200. [Google Scholar]
- Tyerman S. D., Findlay G. P., Paterson G. J. Inward membrane current in Chara inflata. I. A voltage and time-dependent Cl- component. J. Membr. Biol. 1986;89:139–152. [Google Scholar]
- Tyerman S. D., Findlay G. P., Paterson G. J. Inward membrane current in Chara inflata. II. Effects of pH, Cl- channel blockers and NH4+ and significance for the hyperpolarized state. J. Membr. Biol. 1986;89:153–161. [Google Scholar]
- Tyerman S. D., Findlay G. P. Current-voltage curves of single Cl- channels which coexist with two types of K+ channel in tonoplast of Chara corallina. J. Exp. Bot. 1989;40:105–117. [Google Scholar]
- Van Sambeek J. W., Pickard B. G. Mediation of rapid electrical metabolic, transpirational and photosynthetic changes by factors released from wounds. I. Variation potentials and putative action potentials in intact plants. Canadian J. Bot. 1976;54:2642–2650. [Google Scholar]
- Williamson R. E., Ashley C. C. Free Ca2+ and cytoplasmatic streaming in the alga Chara. Nature. 1982;296:647–651. doi: 10.1038/296647a0. [DOI] [PubMed] [Google Scholar]
- Zawadzki T. Action potentials in Lupinus angustifoliusL. shoots. V. Spread of excitation in the stem, leaves and root. J. Exp. Bot. 1980;31:1371–1377. [Google Scholar]
- Zawadzki T., Davies E. D., Tr Characteristics of action potentials in Heliantus annuus. Physiol. Plant. 1991;83:601–604. [Google Scholar]


