Abstract
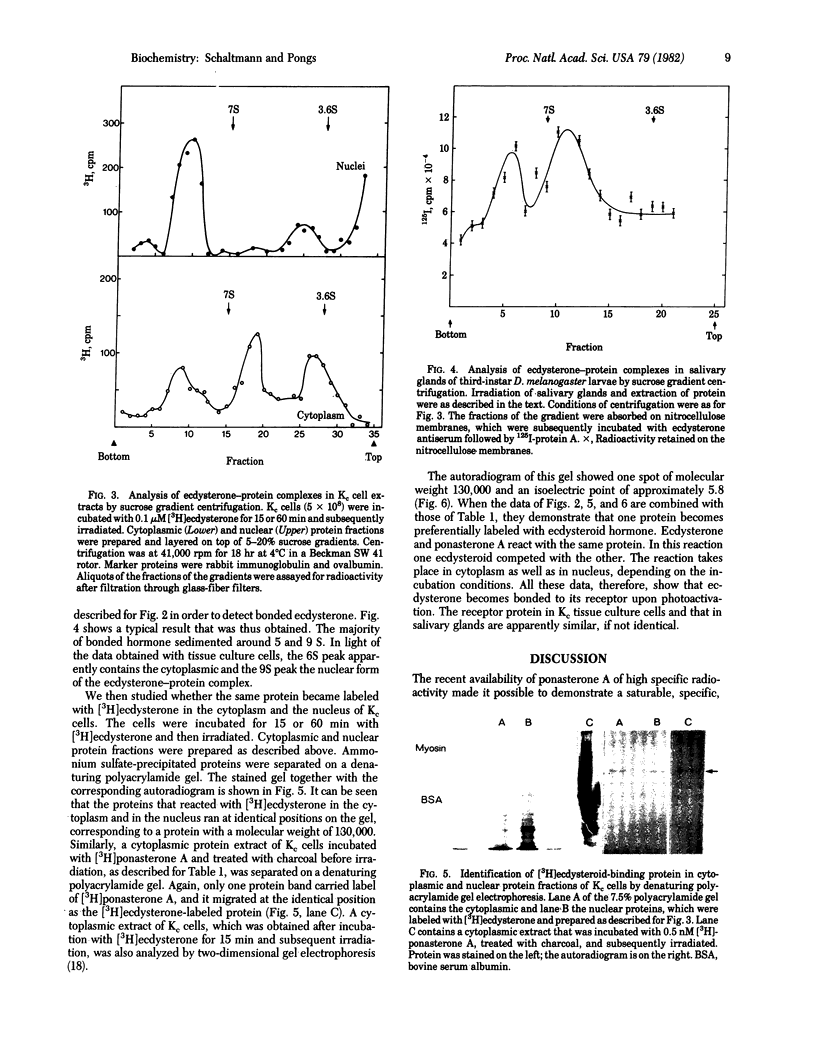
Salivary glands of third-instar larvae of Drosophila melanogaster as well as Drosophila Kc tissue culture cells have been irradiated in the presence of ecdysterone. Irradiation covalently links ecdysterone to a single cellular protein, which is similar, if not identical, in salivary glands and in Kc cells. This protein has a molecular weight of 130,000 and it has the characteristics of a typical hormone-receptor molecule in terms of hormone-binding properties, translocation into the nucleus, and sedimentation characteristics. The yield of the photoinduced bonding of ecdysterone to receptor protein is around 15%. Ponasterone A competed with ecdysterone for the bonding. Also, ponasterone A itself reacted upon photoactivation with the β-ecdysterone receptor protein in Drosophila tissue culture cells. We have previously shown that ecdysterone can be bonded upon irradiation to specific hormone-controlled puffs of polytene chromosomes of D. melanogaster third-instar larvae [Gronemeyer, H. & Pongs, O. (1980) Proc. Natl. Acad. Sci. USA 77, 2108-2112]. Because we have now identified the molecular target of the ecdysterone photoreaction, these data show that a hormone-receptor complex translocates to the nucleus and directly binds to the genes, which are under hormonal control. A quantitative assay of hormone-receptor complex in Kc cells before and after hormone stimulation showed that ecdysterone does not regulate the synthesis and the available amount of its receptor. It was also observed that the translocated hormone-receptor complex resides in the nucleus as long as the hormone is present in the tissue culture medium.
Keywords: steroid hormones, blotting technique, irradiation
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. H., Gotchel B. V. Histones of polytene and nonpolytene nuclei of Drosophila melanogaster. J Biol Chem. 1971 Mar 25;246(6):1841–1848. [PubMed] [Google Scholar]
- Courgeon A. M. Action of insect hormones at the cellular level. Morphological changes of a diploid cell line of Drosophila melanogaster, treated with ecdysone and several analogues in vitro. Exp Cell Res. 1972 Oct;74(2):327–336. doi: 10.1016/0014-4827(72)90384-9. [DOI] [PubMed] [Google Scholar]
- Echalier G., Ohanessian A. In vitro culture of Drosophila melanogaster embryonic cells. In Vitro. 1970 Nov-Dec;6(3):162–172. doi: 10.1007/BF02617759. [DOI] [PubMed] [Google Scholar]
- GRACE T. D. Establishment of four strains of cells from insect tissues grown in vitro. Nature. 1962 Aug 25;195:788–789. doi: 10.1038/195788a0. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Pongs O. Localization of ecdysterone on polytene chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2108–2112. doi: 10.1073/pnas.77.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maroy P., Dennis R., Beckers C., Sage B. A., O'Connor J. D. Demonstration of an ecdysteroid receptor in a cultured cell line of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6035–6038. doi: 10.1073/pnas.75.12.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Samal B., Worcel A., Louis C., Schedl P. Chromatin structure of the histone genes of D. melanogaster. Cell. 1981 Feb;23(2):401–409. doi: 10.1016/0092-8674(81)90135-5. [DOI] [PubMed] [Google Scholar]
- Schaltmann K., Pongs O. A simple procedure for blotting of proteins to study antibody specificity and antigen structure. Hoppe Seylers Z Physiol Chem. 1980;361(2):207–210. [PubMed] [Google Scholar]
- Schrader W. T. Methods for extraction and quantification of receptors. Methods Enzymol. 1975;36:187–211. doi: 10.1016/s0076-6879(75)36020-5. [DOI] [PubMed] [Google Scholar]
- Taylor C. A., Jr, Smith H. E., Danzo B. J. Photoaffinity labeling of rat androgen binding protein. Proc Natl Acad Sci U S A. 1980 Jan;77(1):234–238. doi: 10.1073/pnas.77.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]
- Yund M. A., King D. S., Fristrom J. W. Ecdysteroid receptors in imaginal discs of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6039–6043. doi: 10.1073/pnas.75.12.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]