Abstract
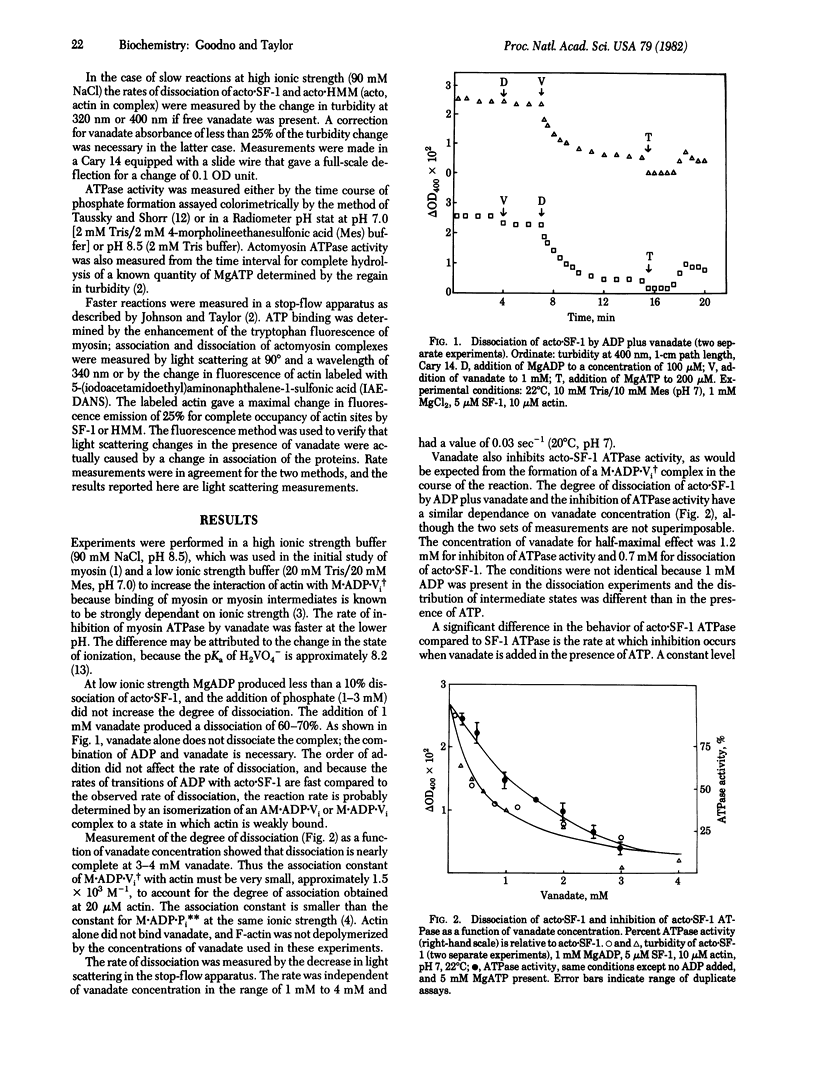
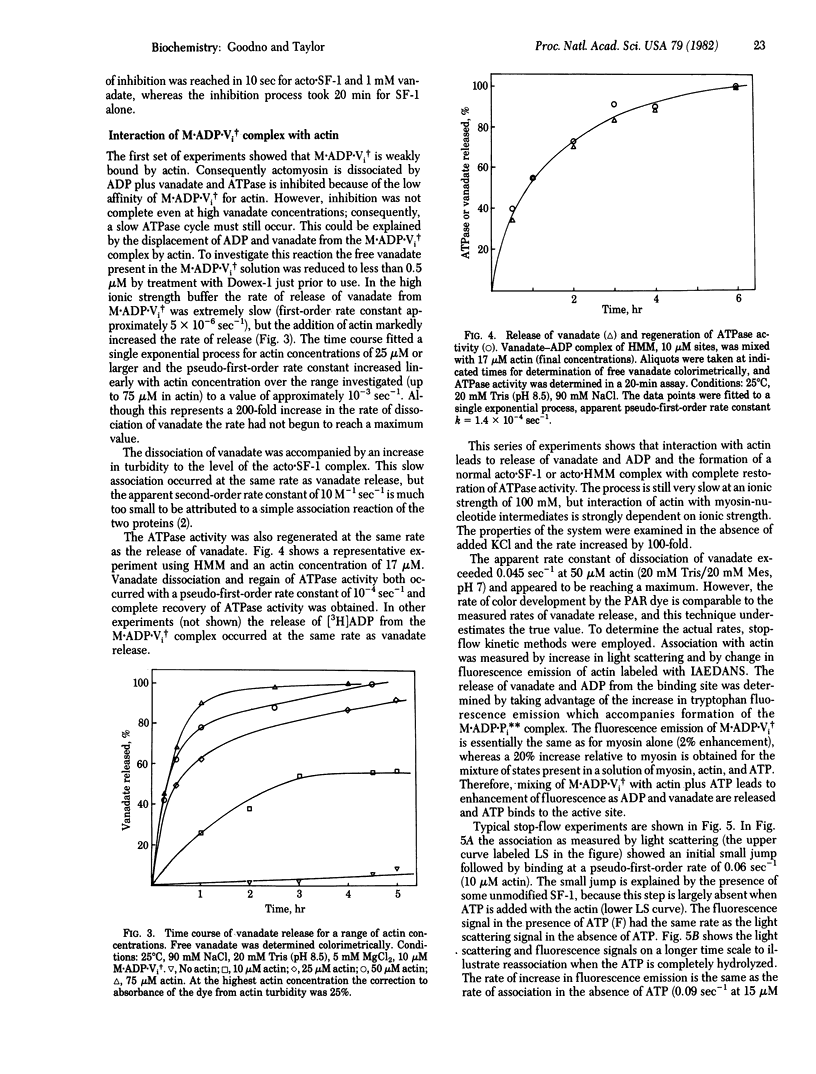
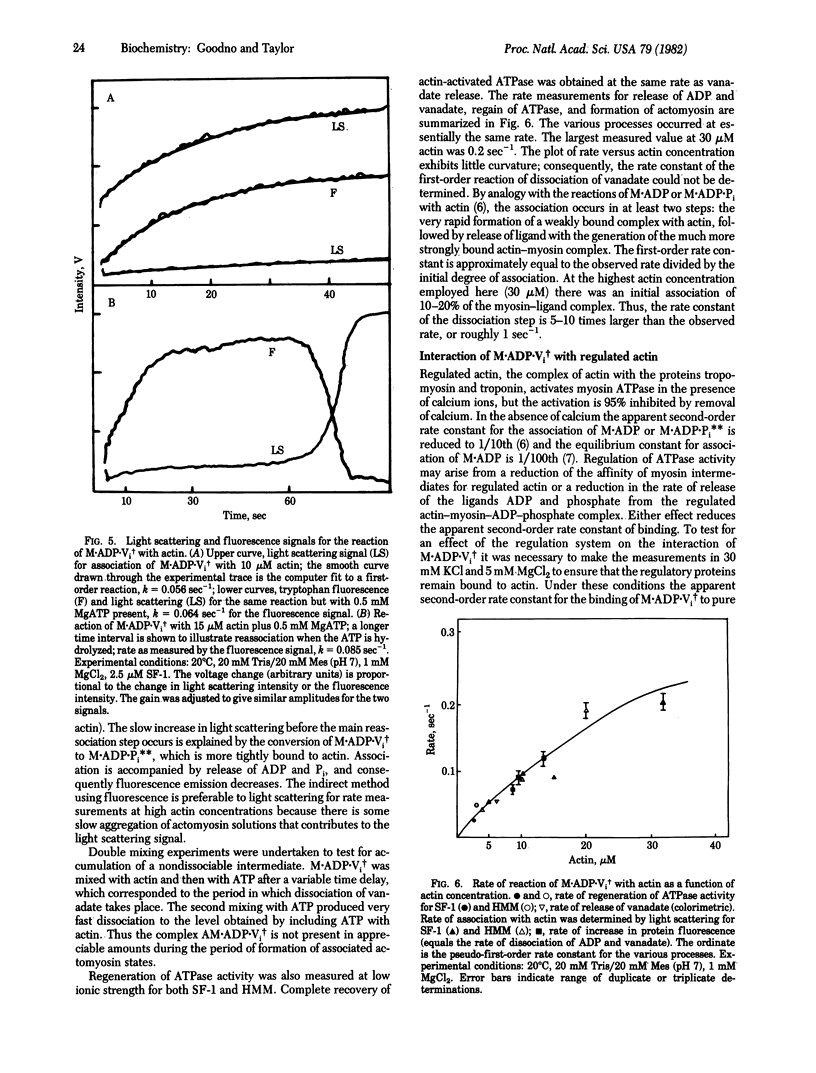
Actin-myosin subfragment-1 (SF-1) or actin-heavy meromyosin is dissociated by the binding of ADP and vanadate (Vi) under conditions such that ADP alone does not dissociate the complex. The association constant of the stable complex M.ADP.Vi, in which M indicates myosin [Goodno, C. C. (1979) Proc. Natl. Acad. Sci. USA 76, 2620-2624] with actin is smaller than the average association constant of the intermediate states of the actin-SF-1 ATPase cycle. Actin-SF-1 ATPase activity is 90% inhibited by ADP plus vanadate. The reaction of actin with M.ADP.Vi produces a slow release of ADP and vanadate and quantitative recovery of ATPase activity. The rate of dissociation of ligands was almost linear in actin concentration; consequently, the rate constant of dissociation could only be roughly estimated as 0.5-1 sec-1. The rate of dissociation of ADP and vanadate is thus increased by a factor of 10(5) compared to M.ADP.Vi. The rate of release of ligands by regulated actin (actin-tropomyosin-troponin) was reduced to 1/10th to 1/20th by removal of calcium ion. Therefore the M.ADP.Vi complex has the properties of a more stable analogue of the myosin-ADP-phosphate complex that is generated in the normal ATPase cycle. The activation of ligand release (ratio of rate of dissociation of ADP and vanadate from actomyosin relative to myosin) is much larger than the activation of myosin ATPase by actin, whereas the actual rates of the reactions are much slower.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goodno C. C. Inhibition of myosin ATPase by vanadate ion. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2620–2624. doi: 10.1073/pnas.76.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody R. S., Hofmann W., Mannherz G. H. The binding constant of ATP to myosin S1 fragment. Eur J Biochem. 1977 Sep;78(2):317–324. doi: 10.1111/j.1432-1033.1977.tb11742.x. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock S. E. Regulation of muscle contraction. Effect of calcium on the affinity of troponin for actin and tropomyosin. Biochemistry. 1973 Jun 19;12(13):2509–2513. doi: 10.1021/bi00737a022. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Taylor E. W. Intermediate states of subfragment 1 and actosubfragment 1 ATPase: reevaluation of the mechanism. Biochemistry. 1978 Aug 22;17(17):3432–3442. doi: 10.1021/bi00610a002. [DOI] [PubMed] [Google Scholar]
- Sleep J. A., Hutton R. L. Actin mediated release of ATP from a myosin-ATP complex. Biochemistry. 1978 Dec 12;17(25):5423–5430. doi: 10.1021/bi00618a016. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7209–7213. doi: 10.1073/pnas.77.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]



