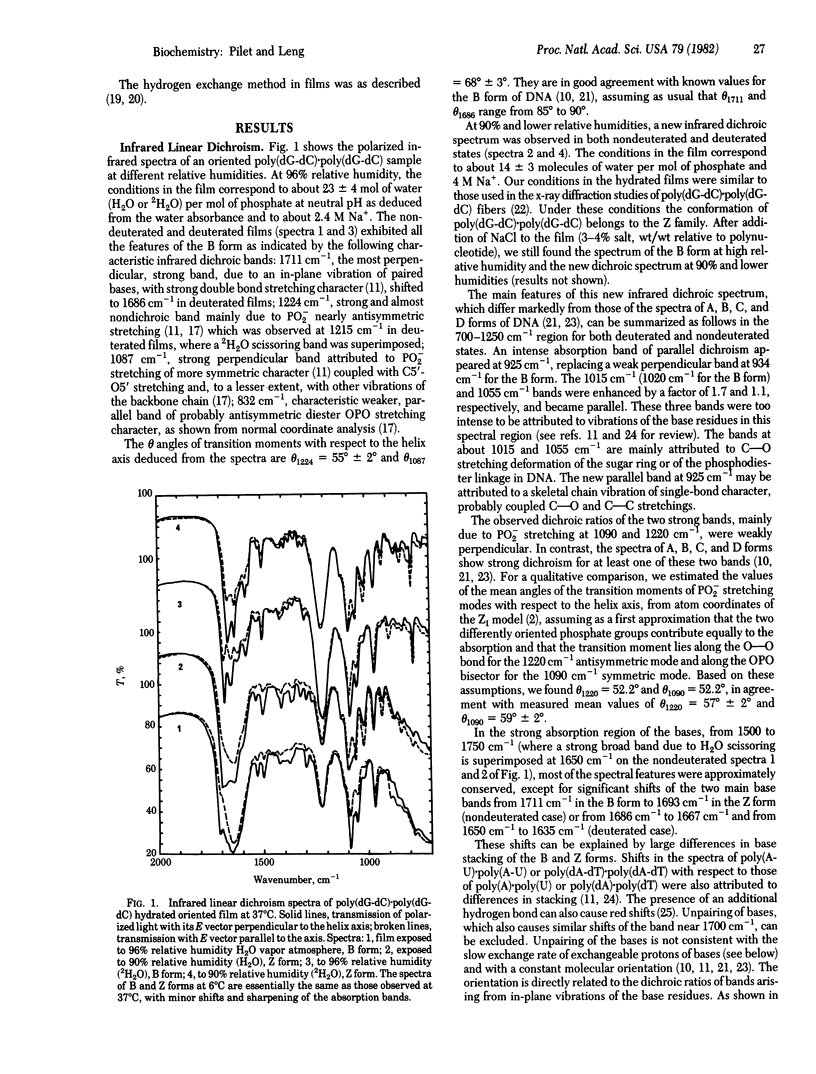
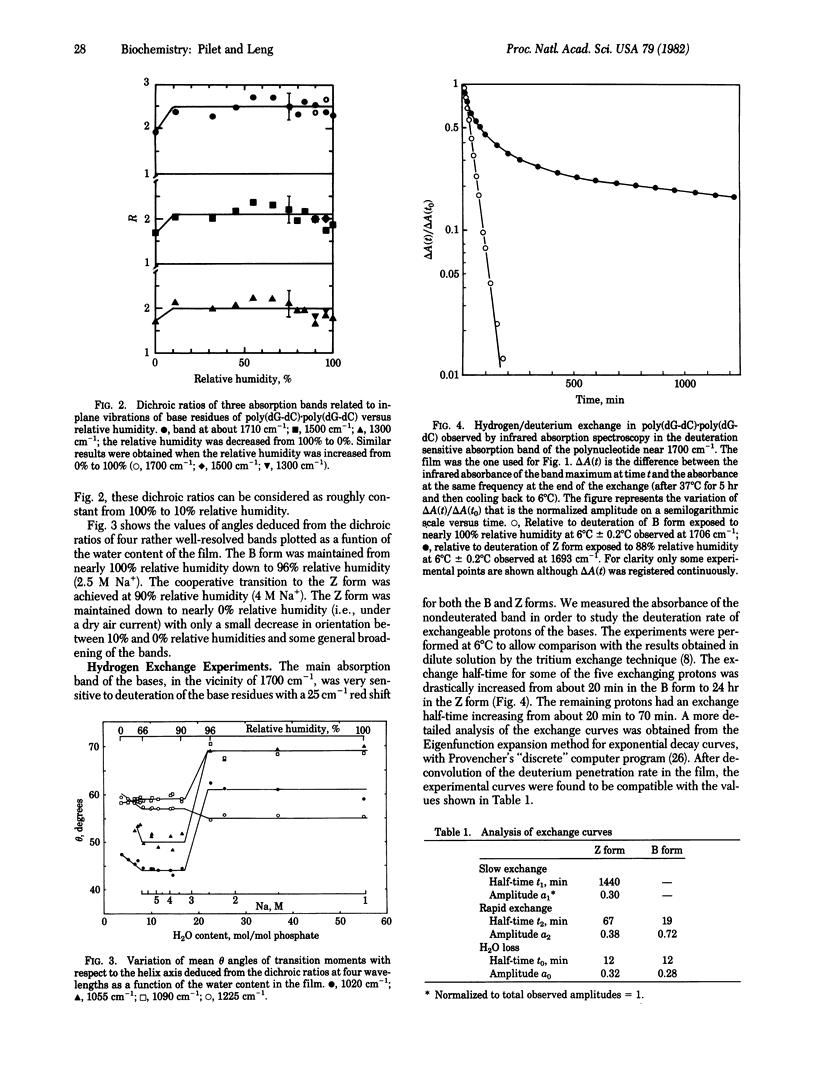
Abstract
The structures of B and Z forms of poly(dG-dC).poly(dG-dC) in oriented hydrated films have been investigated by infrared spectroscopy. First, the infrared linear dichroism spectrum of poly(dG-dC).poly(dG-dC) in B form (high relative humidity and 2 M Na+) was recorded. The spectrum of the Z form was recorded at a lower relative humidity and higher sodium content (4 M). The experimental conditions were similar to those used in x-ray diffraction studies of left-handed poly(dG-dC).poly(dG-dC) fibers. A qualitative agreement was found between the calculated values of the angles characteristic of the orientation of PO-2 groups with respect to the helical axis in the ZI form and the experimental values. The infrared spectra of B and Z form and the experimental values. The infrared spectra of B and Z forms show some significant differences in H2O and 2H2O. The deuteration rates of exchangeable protons involved in hydrogen bonds between guanine and cytosine residues were deduced from the changes in absorbance near 1700 cm-1. In the B form, the exchange half-time is of the order of 20 min. In the Z form, the exchange half-time of some protons is very slow, of the order of 24 hr. Because of the similarity between these values and those previously reported for the high-salt and low-salt form of poly(dG-dC).poly(dG-dC) in solution, we conclude that the high-salt form of poly(dG-dC).poly(dG-dC) in solution belongs to the Z family.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W. Refinement of the structure of B-DNA and implications for the analysis of x-ray diffraction data from fibers of biopolymers. J Mol Biol. 1973 Dec 5;81(2):93–105. doi: 10.1016/0022-2836(73)90182-4. [DOI] [PubMed] [Google Scholar]
- Beetz C. P., Jr, Ascarelli G., Arnott S. A reinterpretation of the infrared linear dichroism of oriented nucleic acid films and a calculation of some effective partial changes on the ribose phosphate backbone. Biophys J. 1979 Oct;28(1):15–26. doi: 10.1016/S0006-3495(79)85155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahms J., Pilet J., Phuong Lan T. T., Hill L. R. Direct evidence of the C-like form of sodium deoxyribonucleate. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3352–3355. doi: 10.1073/pnas.70.12.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Lu K. C., Van Zandt L. L., Prohofsky E. W. Displacements of backbone vibrational modes of A-DNA and B-DNA. Biophys J. 1979 Oct;28(1):27–32. doi: 10.1016/S0006-3495(79)85156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILES H. T. A proposed interpretation of infra-red spectral changes occurring upon the interaction of polynucleotides. Nature. 1959 Jun 27;183:1814–1814. doi: 10.1038/1831814a0. [DOI] [PubMed] [Google Scholar]
- Mitra C. K., Sarma M. H., Sarma R. H. Left-handed deoxyribonucleic acid double helix in solution. Biochemistry. 1981 Mar 31;20(7):2036–2041. doi: 10.1021/bi00510a046. [DOI] [PubMed] [Google Scholar]
- Nairn J. A., Friesner R., Frank H. A., Sauer K. A new approach to the theory of linear dichroism in partially ordered systems. Application to reaction centers and whole cells of photosynthetic bacteria. Biophys J. 1980 Nov;32(2):733–753. doi: 10.1016/S0006-3495(80)85013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet J., Blicharski J., Brahms J. Conformations and structural transitions in polydeoxynucleotides. Biochemistry. 1975 May 6;14(9):1869–1876. doi: 10.1021/bi00680a011. [DOI] [PubMed] [Google Scholar]
- Pilet J., Szabo A. G., Maurizot J. C. Hydrogen exchange in hydrated films of proteins. Application to the E. coli lac repressor core. Biophys Chem. 1980 Dec;12(3-4):279–284. doi: 10.1016/0301-4622(80)80005-6. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Ramstein J., Leng M. Salt-dependent dynamic structure of poly(dG-dC) x poly(dG-dC). Nature. 1980 Nov 27;288(5789):413–414. doi: 10.1038/288413a0. [DOI] [PubMed] [Google Scholar]
- Sage E., Leng M. Conformational changes of poly(dG-dC) . poly(dG-dC) modified by the carcinogen N-acetoxy-N-acetyl-2-aminofluorene. Nucleic Acids Res. 1981 Mar 11;9(5):1241–1250. doi: 10.1093/nar/9.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo H., Wooten J. B., Zimmerman S. B. Geometry of the phosphodiester backbone in the A form of deoxyribonucleic acid determined by phosphorus-31 nuclear magnetic resonance spectroscopy. Biochemistry. 1981 Feb 17;20(4):745–750. doi: 10.1021/bi00507a013. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]



