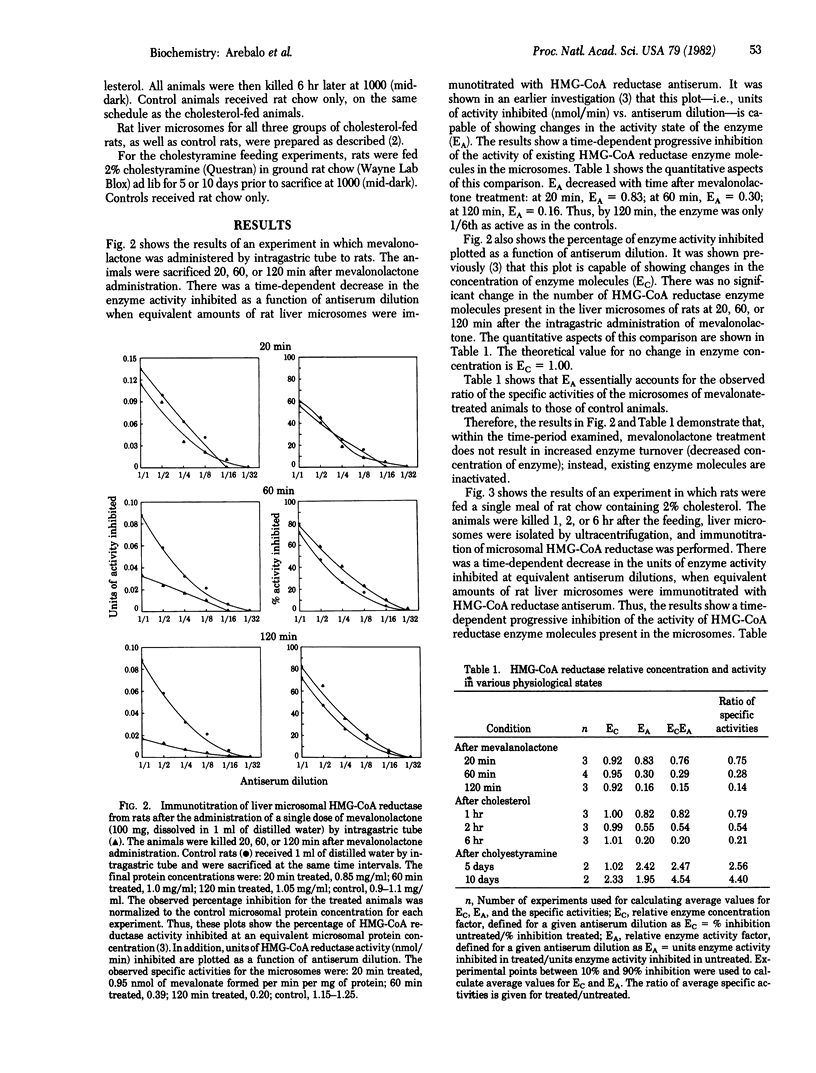
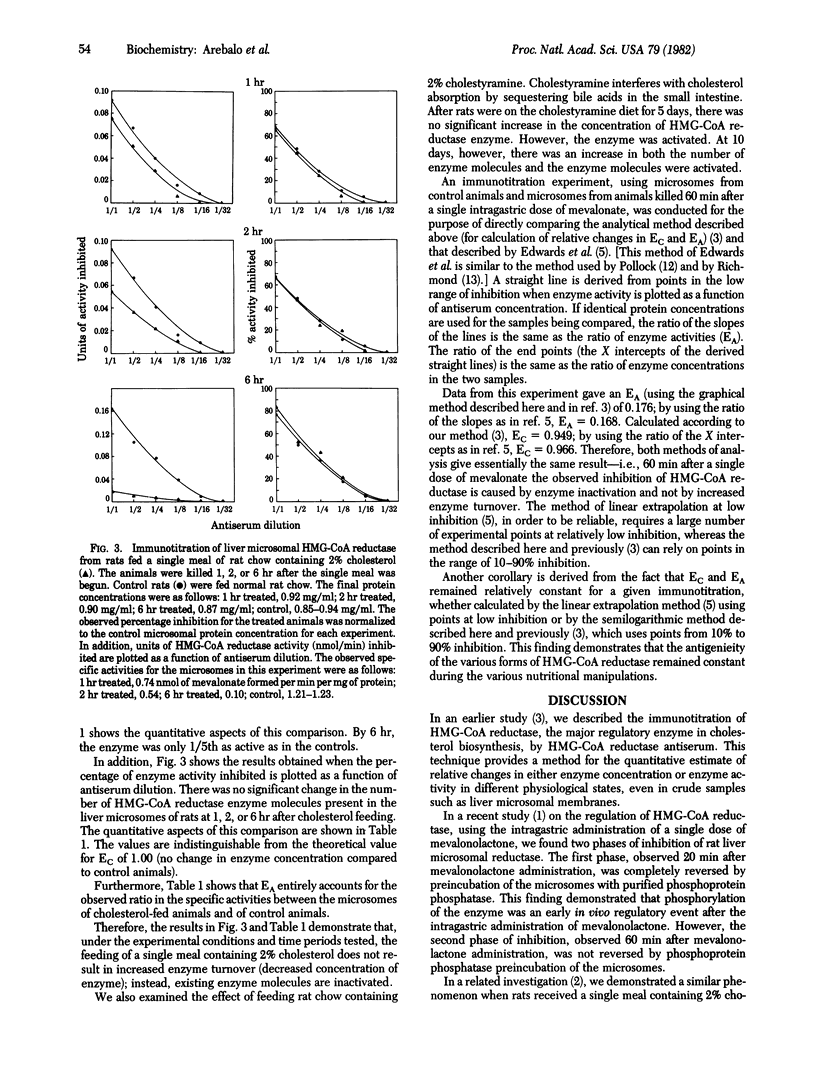
Abstract
In recent studies using either a single dose of mevalonolactone administered by intragastric tube or a single meal containing 2% cholesterol, it was demonstrated that rat liver hydroxymethylglutaryl-coenzyme A reductase [mevalonate: NADP+ oxidoreductase (CoA-acylating), EC 1.1.1.34] (HMG-CoA reductase) the major regulatory enzyme in cholesterol biosynthesis, is subject to two phases of inhibition. The first phase of inhibition is explained by in vivo phosphorylation of the enzyme; however, the nature of the second phase of inhibition remained obscure. The present study tested two possible explanations for this second phase of inhibition--increased enzyme turnover leading to a decreased concentration of HMG-CoA reductase molecules, and further inactivation of existing enzyme molecules. The results with the technique of immunotitration of HMG-CoA reductase show that, in short-term studies conducted up to 2 hr after the administration of a single dose of mevalonolactone or up to 6 hr after a single meal of rat chow containing 2% cholesterol, the in vivo regulation of rat liver HMG-CoA reductase during the first half of the dark period does not occur by increased enzyme turnover but, instead, existing enzyme is further inactivated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arebalo R. E., Hardgrave J. E., Noland B. J., Scallen T. J. In vivo regulation of rat liver 3-hydroxy-3-methylglutaryl-coenzyme A reductase: enzyme phosphorylation as an early regulatory response after intragastric administration of mevalonolactone. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6429–6433. doi: 10.1073/pnas.77.11.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arebalo R. E., Hardgrave J. E., Scallen T. J. The in vivo regulation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase. Phosphorylation of the enzyme as an early regulatory response following cholesterol feeding. J Biol Chem. 1981 Jan 25;256(2):571–574. [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr In vitro and in vivo phosphorylation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase and its modulation by glucagon. J Biol Chem. 1980 Sep 25;255(18):8541–8545. [PubMed] [Google Scholar]
- Edwards P. A., Gould R. G. Dependence of the circadian rhythm of hepatic beta-hydroxy-beta-methylglutaryl coenzyme A on ribonucleic acid synthesis. A possible second site of inhibition by dietary cholesterol. J Biol Chem. 1974 May 10;249(9):2891–2896. [PubMed] [Google Scholar]
- Edwards P. A., Lemongello D., Kane J., Shechter I., Fogelman A. M. Properties of purified rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and regulation of enzyme activity. J Biol Chem. 1980 Apr 25;255(8):3715–3725. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A. Méthode permettant l'étude conjuguée des proprietés électrophorétiques et immunochimiques d'un mélange de protéines; application au sérum sanguin. Biochim Biophys Acta. 1953 Jan;10(1):193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- Hardgrave J. E., Heller R. A., Herrera M. G., Scallen T. J. Immunotitration of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in various physiological states. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3834–3838. doi: 10.1073/pnas.76.8.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M., Rudney H. Regulation of rat liver beta-hydroxy-beta-methylglutaryl-CoA reductase activity by cholesterol. Nat New Biol. 1973 Nov 14;246(150):60–61. doi: 10.1038/newbio246060a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. An immunological study of the constitutive and the penicillin-induced penicillinases of Bacillus cereus, based on specific enzyme neutralization by antibody. J Gen Microbiol. 1956 Feb;14(1):90–108. doi: 10.1099/00221287-14-1-90. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. Immunological properties of exopenicillinase synthesized by Bacillus cereus 569/H in the presence of amino acid analogues. Biochem J. 1960 Oct;77:112–121. doi: 10.1042/bj0770112. [DOI] [PMC free article] [PubMed] [Google Scholar]