Abstract
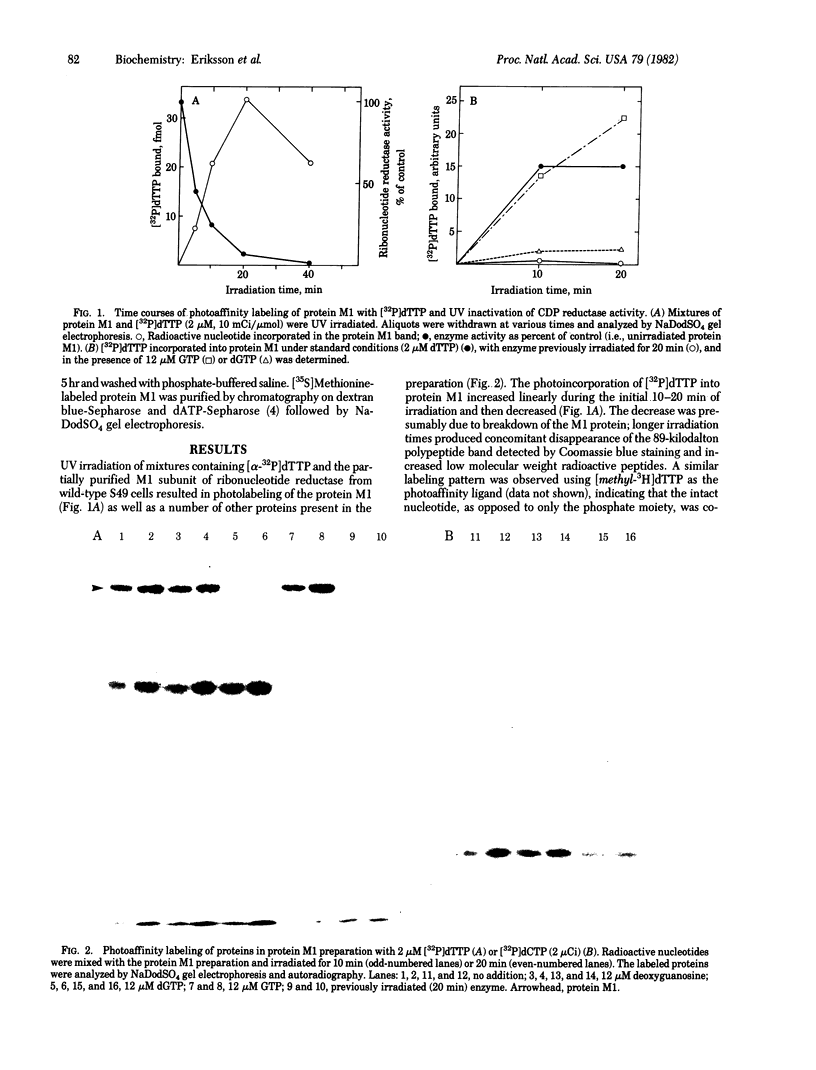
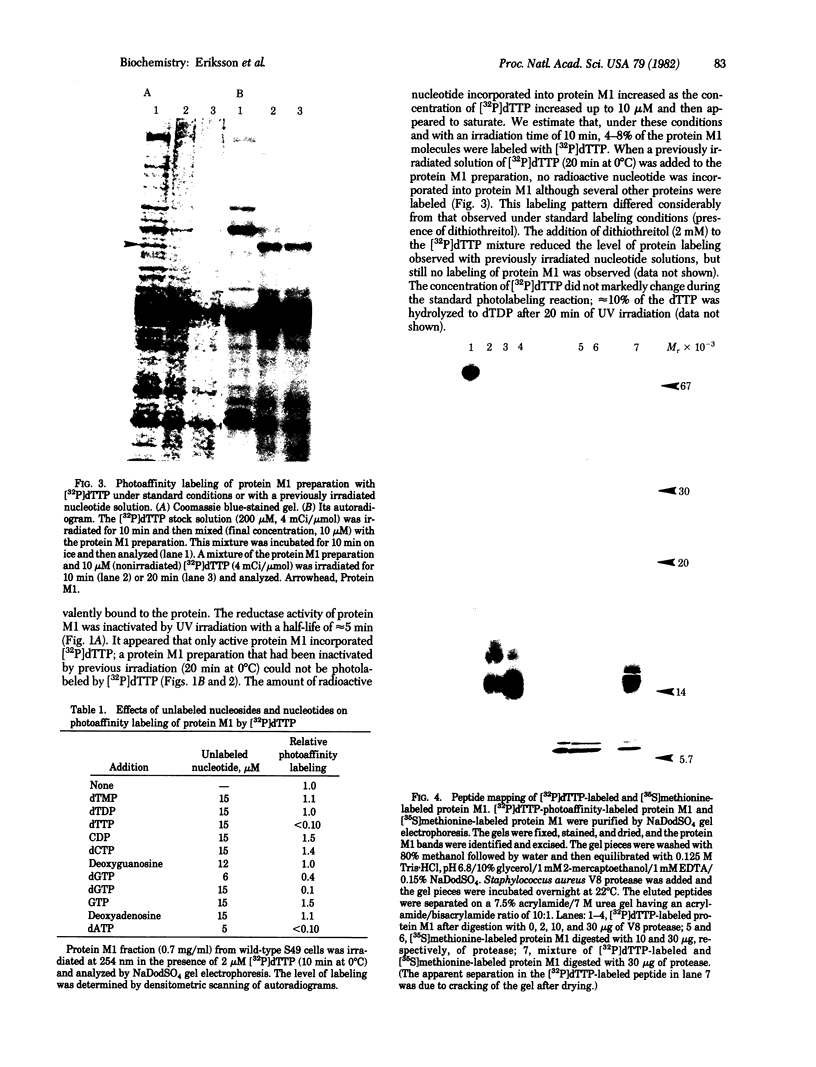
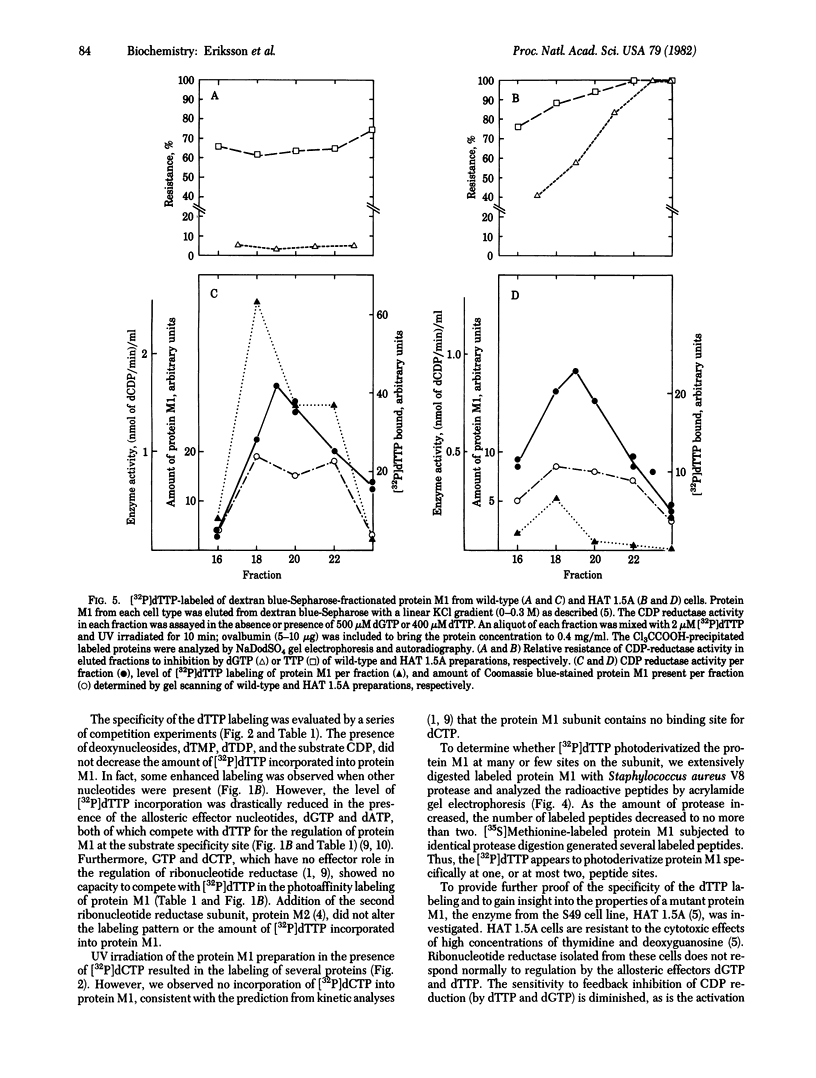
The protein M1 subunit of ribonucleotide reductase contains at least two allosteric nucleotide binding sites that control the capacity of the enzyme to reduce ribonucleotides to the deoxyribonucleotides required for DNA synthesis. Direct photoaffinity labeling of partially purified protein M1 from mouse T-lymphoma (S49) cells was observed after UV irradiation in the presence of dTTP at 0 degrees C. The relative molar incorporation of nucleotide per subunit was 4-8%. Competition experiments showed that the dTTP was bound to an allosteric domain genetically and kinetically defined as the substrate specificity site of the enzyme. An altered protein M1 isolated from a thymidine-resistant mutant cell line showed significantly decreased photoincorporation of dTTP, consistent with the fact that its CDP reductase activity is resistant to feedback inhibition by dTTP. Specific photolabeling of several other proteins with pyrimidine and purine nucleotides was also found, indicating the general usefulness of direct photoaffinity labeling in the study of enzymes involved in nucleotide and nucleic acid metabolism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Engström Y., Eriksson S., Thelander L., Akerman M. Ribonucleotide reductase from calf thymus. Purification and properties. Biochemistry. 1979 Jul 10;18(14):2941–2948. doi: 10.1021/bi00581a004. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Gudas L. J., Clift S. M., Caras I. W., Ullman B., Martin D. W., Jr Evidence for genetically independent allosteric regulatory domains of the protein M1 subunit of mouse ribonucleotide reductase. J Biol Chem. 1981 Oct 10;256(19):10193–10197. [PubMed] [Google Scholar]
- Eriksson S., Gudas L. J., Ullman B., Clift S. M., Martin D. W., Jr DeoxyATP-resistant ribonucleotide reductase of mutant mouse lymphoma cells. Evidence for heterozygosity for the protein M1 subunits. J Biol Chem. 1981 Oct 10;256(19):10184–10188. [PubMed] [Google Scholar]
- Eriksson S., Thelander L., Akerman M. Allosteric regulation of calf thymus ribonucleoside diphosphate reductase. Biochemistry. 1979 Jul 10;18(14):2948–2952. doi: 10.1021/bi00581a005. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maruta H., Korn E. D. Direct photoaffinity labeling by nucleotides of the apparent catalytic site on the heavy chains of smooth muscle and Acanthamoeba myosins. J Biol Chem. 1981 Jan 10;256(1):499–502. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Caras I. W., Eriksson S., Weinberg G. L., Wormsted M. A., Martin D. W., Jr Demonstration of normal and mutant protein M1 subunits of deoxyGTP-resistant ribonucleotide reductase from mutant mouse lymphoma cells. J Biol Chem. 1981 Oct 10;256(19):10189–10192. [PubMed] [Google Scholar]
- Yue V. T., Schimmel P. R. Direct and specific photochemical cross-linking of adenosine 5'-triphosphate to an aminoacyl-tRNA synthetase. Biochemistry. 1977 Oct 18;16(21):4678–4684. doi: 10.1021/bi00640a023. [DOI] [PubMed] [Google Scholar]