Abstract
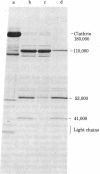
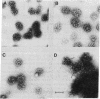
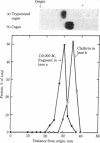
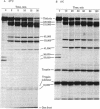
Triskelions, trimeric complexes of clathrin and associated light chains, are the assembly units of clathrin coats [Ungewickell, E. & Branton, D. (1981) Nature (London) 289, 420-422; Kirchhausen, T. & Harrison, S. C. (1981) Cell 23, 755-761]. We report here that triskelions whose outer arms have been removed by trypsin digestion retain the ability to be assembled into coats. These digested timers contain a 110,000 molecular weight domain of clathrin and lack intact light chains.
Full text
PDF
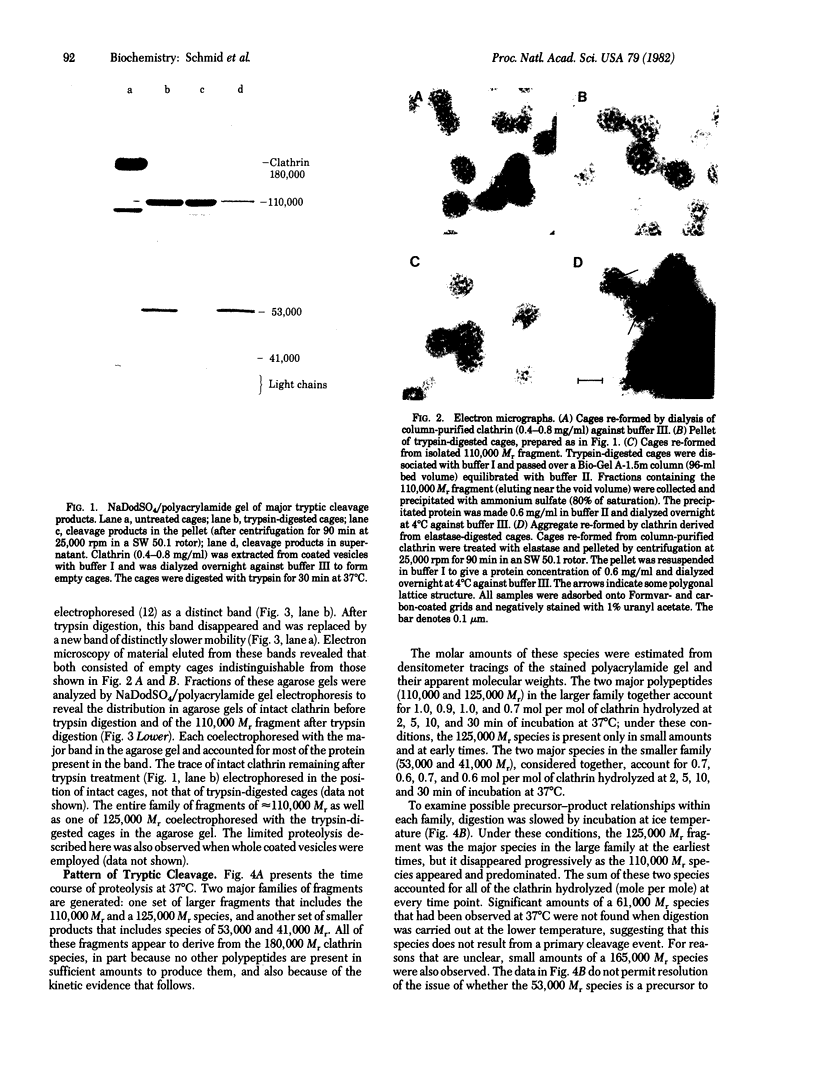
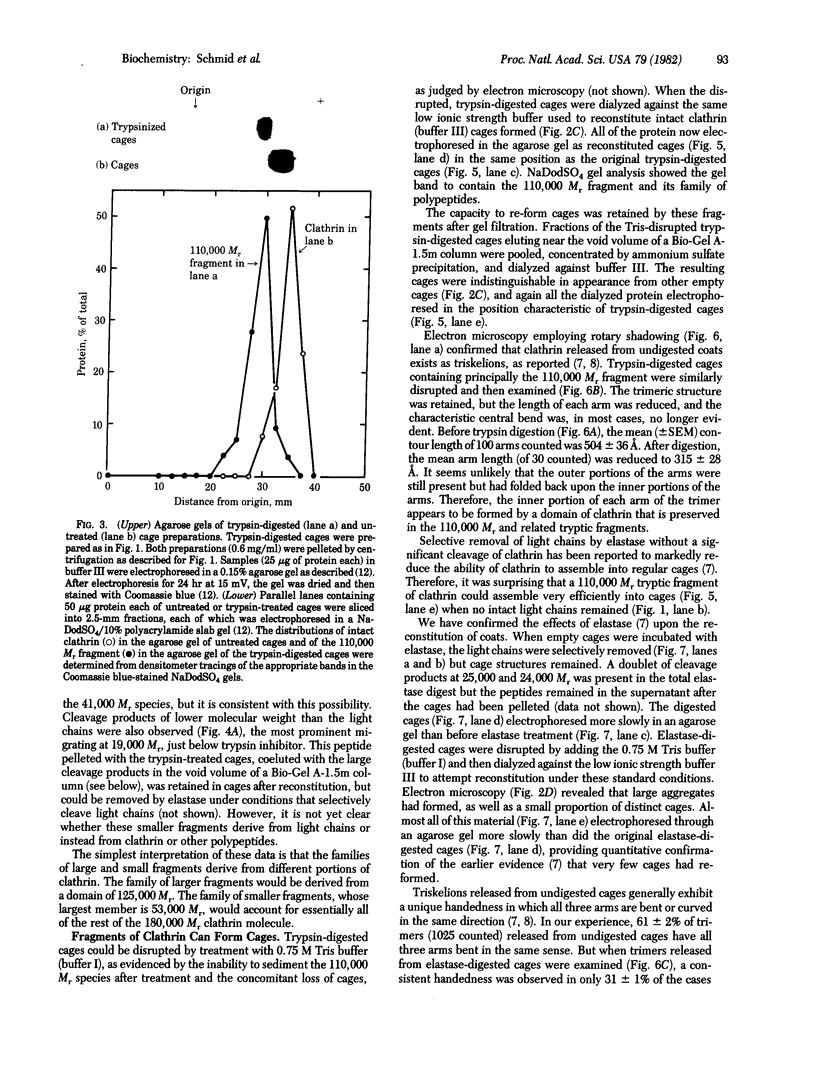


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Keen J. H., Willingham M. C., Pastan I. H. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979 Feb;16(2):303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Harrison S. C. Protein organization in clathrin trimers. Cell. 1981 Mar;23(3):755–761. doi: 10.1016/0092-8674(81)90439-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nandi P. K., Pretorius H. T., Lippoldt R. E., Johnson M. L., Edelhoch H. Molecular properties of the reassembled coat protein of coated vesicles. Biochemistry. 1980 Dec 9;19(25):5917–5921. doi: 10.1021/bi00566a039. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975 Sep 5;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Fine R. E., Luskey B. D., Rothman J. E. Purification of coated vesicles by agarose gel electrophoresis. J Cell Biol. 1981 May;89(2):357–361. doi: 10.1083/jcb.89.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schook W., Puszkin S., Bloom W., Ores C., Kochwa S. Mechanochemical properties of brain clathrin: interactions with actin and alpha-actinin and polymerization into basketlike structures or filaments. Proc Natl Acad Sci U S A. 1979 Jan;76(1):116–120. doi: 10.1073/pnas.76.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Branton D. Rotary shadowing of extended molecules dried from glycerol. J Ultrastruct Res. 1980 May;71(2):95–102. doi: 10.1016/s0022-5320(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Branton D. Assembly units of clathrin coats. Nature. 1981 Jan 29;289(5796):420–422. doi: 10.1038/289420a0. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Roth T. F. Coated vesicles: characterization, selective dissociation, and reassembly. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4394–4398. doi: 10.1073/pnas.75.9.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]