Abstract
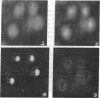
The focusing properties of a magnetic-sector spectrometer are shown to be suitable for forming high-spatial-resolution, energy-filtered transmission electron microscope images. Filtered images of ferritin molecules by using electrons scattered from the characteristic iron M2,3 and carbon K absorption edges clearly distinguish the 75-A iron core and 120-A protein shell. The minimum detectable mass is estimated to be 0.84 X 10(-20) g for Fe for an electron dose of 18 C/cm2 and 99% confidence.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Egerton R. F. Formulae for light-element microanalysis by electron energy-loss spectrometry. Ultramicroscopy. 1978;3(2):243–251. doi: 10.1016/s0304-3991(78)80031-x. [DOI] [PubMed] [Google Scholar]
- Hainfeld J., Isaacson M. The use of electron energy loss spectroscopy for studying membrane architecture: a preliminary report. Ultramicroscopy. 1978;3(1):87–95. doi: 10.1016/s0304-3991(78)80011-4. [DOI] [PubMed] [Google Scholar]
- Isaacson M. S., Crewe A. V. Electron microspectroscopy. Annu Rev Biophys Bioeng. 1975;4(00):165–184. doi: 10.1146/annurev.bb.04.060175.001121. [DOI] [PubMed] [Google Scholar]
- Isaacson M. The microanalysis of light elements using transmitted energy loss electrons. Ultramicroscopy. 1975 Jul;1(1):33–52. doi: 10.1016/s0304-3991(75)80006-4. [DOI] [PubMed] [Google Scholar]
- Massover W. H. The ultrastructure of ferritin macromolecules. III. Mineralized iron in ferritin is attached to the protein shell. J Mol Biol. 1978 Aug 25;123(4):721–726. doi: 10.1016/0022-2836(78)90218-8. [DOI] [PubMed] [Google Scholar]
- Shuman H. Correction of the second-order aberrations of uniform field magnetic sectors. Ultramicroscopy. 1980;5(1):45–53. doi: 10.1016/0304-3991(80)90010-8. [DOI] [PubMed] [Google Scholar]
- Shuman H. Parallel recording of electron energy loss spectra. Ultramicroscopy. 1981;6(2):163–167. doi: 10.1016/0304-3991(81)90056-5. [DOI] [PubMed] [Google Scholar]
- Shuman H., Somlyo A. P. Electron probe x-ray analysis of single ferritin molecules. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1193–1195. doi: 10.1073/pnas.73.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H., Stewart M. Electron probe analysis of muscle and X-ray mapping of biological specimens with a field emission gun. Scan Electron Microsc. 1979;(2):711–722. [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M., Somlyo A. P., Somlyo A. V., Shuman H., Lindsay J. A., Murrell W. G. Distribution of calcium and other elements in cryosectioned Bacillus cereus T spores, determined by high-resolution scanning electron probe x-ray microanalysis. J Bacteriol. 1980 Jul;143(1):481–491. doi: 10.1128/jb.143.1.481-491.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]