Abstract
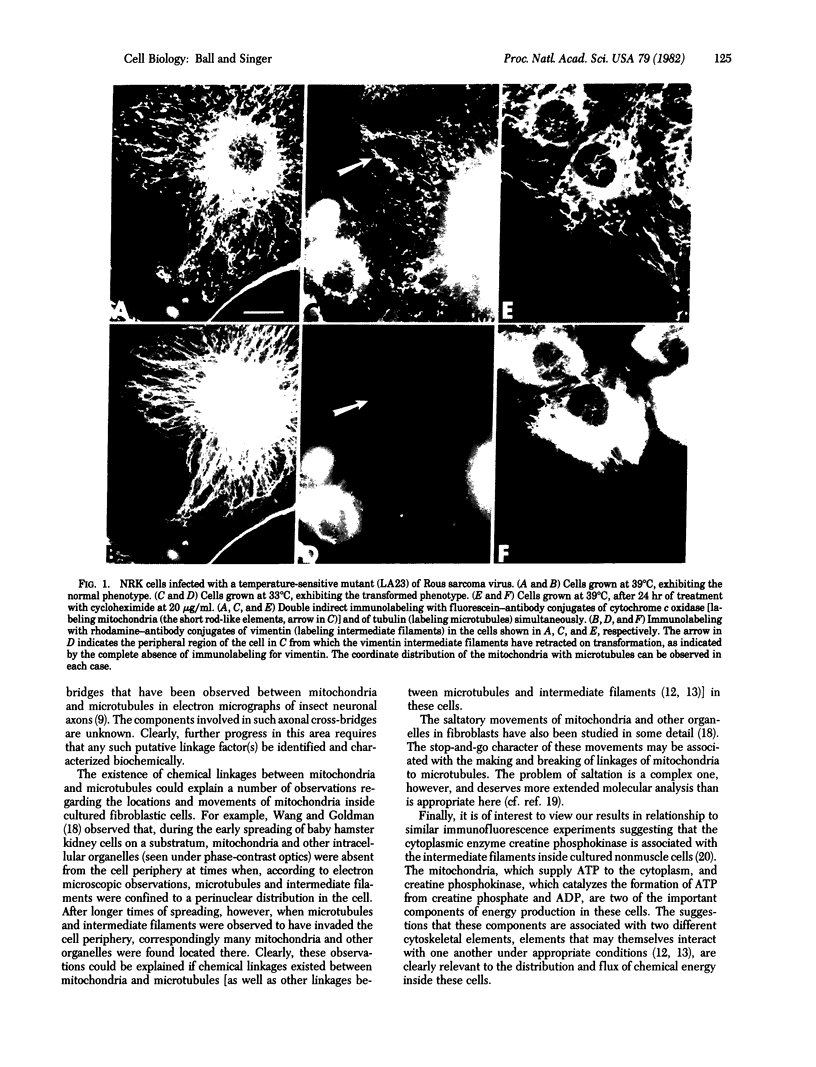
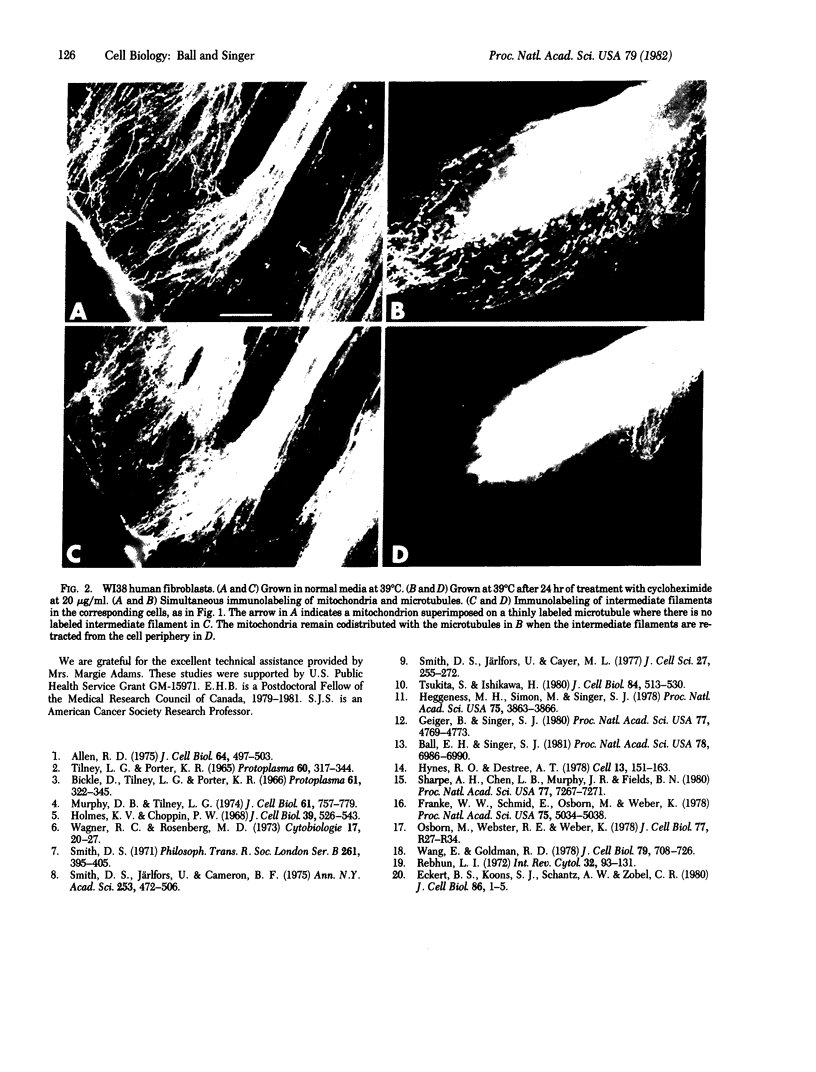
Triple-immunofluorescence experiments with antibodies to cytochrome c oxidase, tubulin, and vimentin have been used to immunolabel the mitochondria, microtubules, and intermediate filaments inside the same cultured fibroblasts. In particular, fibroblasts were immunolabeled after they had either been transformed by infection with Rous sarcoma virus or given long-term treatment with cycloheximide. These treatments induced redistribution of the intermediate filaments into a perinuclear arrangement, segregated away from the microtubules, which remained extended to the cell periphery. In such cells, many labeled mitochondria were observed to be codistributed with the peripherally located microtubules. From these results, we infer that an association, probably involving some type of chemical linkage(s), between mitochondria and microtubules exists in these cells that is independent of the intermediate filaments.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D. Evidence for firm linkages between microtubules and membrane-bounded vesicles. J Cell Biol. 1975 Feb;64(2):497–503. doi: 10.1083/jcb.64.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball E. H., Singer S. J. Association of microtubules and intermediate filaments in normal fibroblasts and its disruption upon transformation by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6986–6990. doi: 10.1073/pnas.78.11.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert B. S., Koons S. J., Schantz A. W., Zobel C. R. Association of creatine phosphokinase with the cytoskeleton of cultured mammalian cells. J Cell Biol. 1980 Jul;86(1):1–5. doi: 10.1083/jcb.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Singer S. J. Association of microtubules and intermediate filaments in chicken gizzard cells as detected by double immunofluorescence. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4769–4773. doi: 10.1073/pnas.77.8.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness M. H., Simon M., Singer S. J. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of microtubules in movement and alignment of nuclei in virus-induced syncytia. J Cell Biol. 1968 Dec;39(3):526–543. doi: 10.1083/jcb.39.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Tilney L. G. The role of microtubules in the movement of pigment granules in teleost melanophores. J Cell Biol. 1974 Jun;61(3):757–779. doi: 10.1083/jcb.61.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Webster R. E., Weber K. Individual microtubules viewed by immunofluorescence and electron microscopy in the same PtK2 cell. J Cell Biol. 1978 Jun;77(3):R27–R34. doi: 10.1083/jcb.77.3.r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Chen L. B., Murphy J. R., Fields B. N. Specific disruption of vimentin filament organization in monkey kidney CV-1 cells by diphtheria toxin, exotoxin A, and cycloheximide. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7267–7271. doi: 10.1073/pnas.77.12.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. S., Järlfors U., Cameron B. F. Morphological evidence for the participation of microtubules in axonal transport. Ann N Y Acad Sci. 1975 Jun 30;253:472–506. doi: 10.1111/j.1749-6632.1975.tb19223.x. [DOI] [PubMed] [Google Scholar]
- Smith D. S., Järlfors U., Cayer M. L. Structural cross-bridges between microtubules and mitochondria in central axons of an insect (Periplaneta americana). J Cell Sci. 1977;27:255–272. doi: 10.1242/jcs.27.1.255. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Porter K. R. Studies on microtubules in Heliozoa. I. The fine structure of Actinosphaerium nucleofilum (Barrett), with particular reference to the axial rod structure. Protoplasma. 1965;60(4):317–344. doi: 10.1007/BF01247886. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Ishikawa H. The movement of membranous organelles in axons. Electron microscopic identification of anterogradely and retrogradely transported organelles. J Cell Biol. 1980 Mar;84(3):513–530. doi: 10.1083/jcb.84.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Höer R., Murakami T., Farkas L. Isolierung, Strukturaufklärung und Synthese von 4'5,7-Trihydroxy-3',6-dimethoxyflavon-7-mono-beta-D-glucopyranosid (Jaceosid), einem neuen Flavonglycosid aus den Wurzeln von Centaurea jacea. Chem Ber. 1973;106(1):20–27. doi: 10.1002/cber.19731060104. [DOI] [PubMed] [Google Scholar]
- Wang E., Goldman R. D. Functions of cytoplasmic fibers in intracellular movements in BHK-21 cells. J Cell Biol. 1978 Dec;79(3):708–726. doi: 10.1083/jcb.79.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]