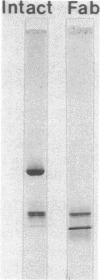
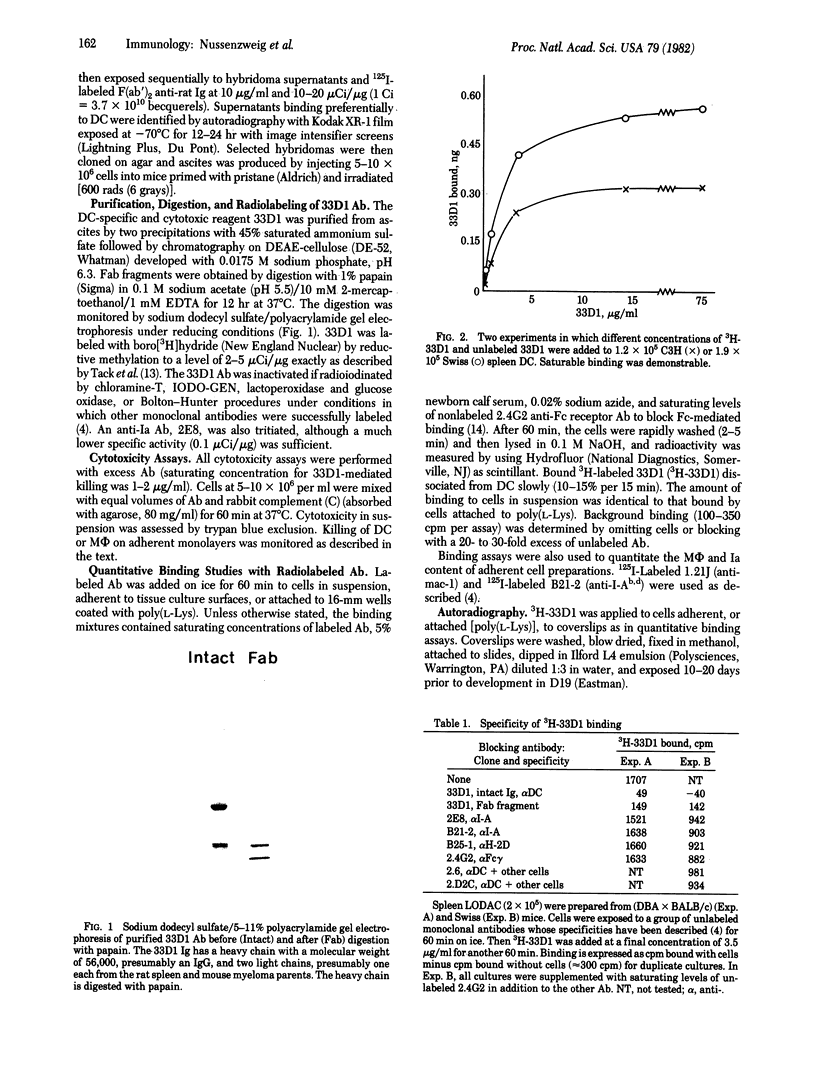
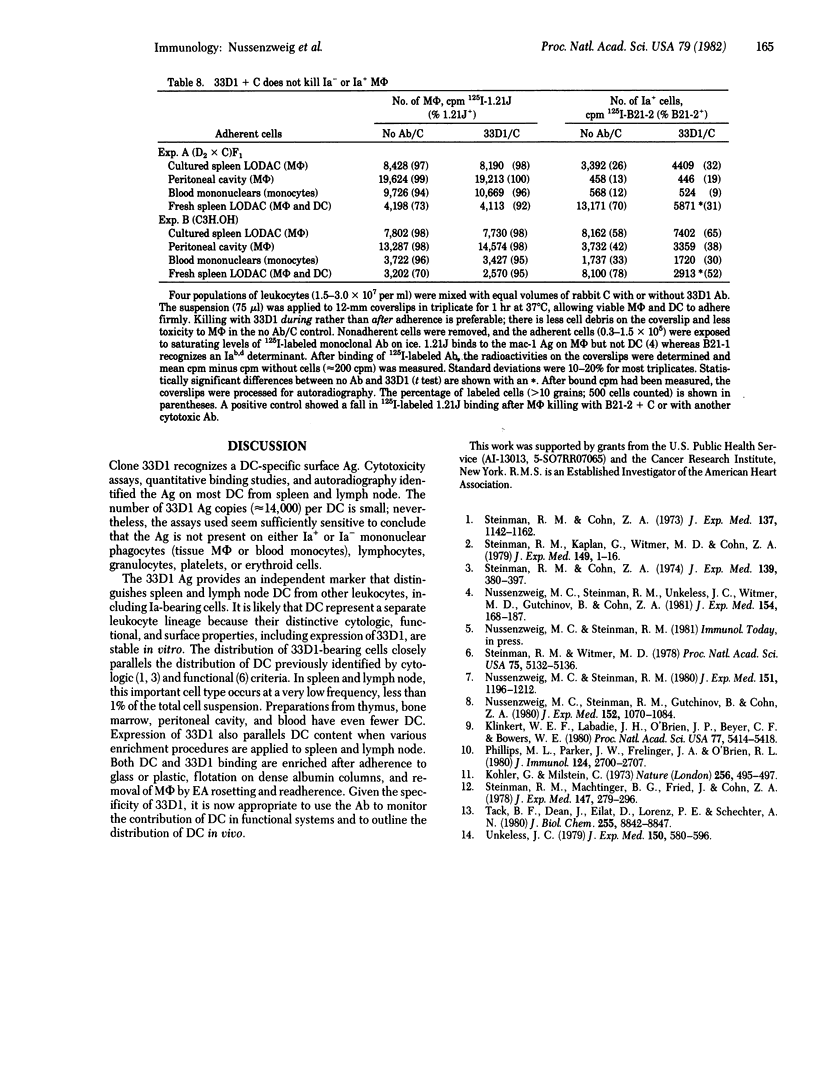
Abstract
Dendritic cells (DC) are a small subpopulation of lymphoid cells with distinctive cytologic features, surface properties, and functions. This report describes the DC-specific antibody (Ab) secreted by clone 33DI. Rat spleen cells immune to mouse DC were fused to the P3U myeloma. Hybrid culture supernatants were screened simultaneously against DC, a macrophage (M phi) cell line, and mitogen-stimulated lymphoblasts. 33DI Ab specifically killed 80-90% of DC from spleen and lymph node, but no other leukocytes, including Ia+ and Ia- M phi (Ia, I-region-associated antigen,). Quantitative binding studies with 5H-labeled 33D1 Ab showed that DC had an average of 14,000 binding sites per cell. Binding to DC was inhibited with Fab fragment of 33D1 Ab but not with a panel of other monoclonal antibodies, including anti-Ia Ab. Adherence and flotation procedures that enriched for DC enriched for 3H-labeled 33D1 Ab binding in parallel. 33D1 antigen was not detectable on: M phi from spleen, peritoneal cavity, and blood; three M phi cell lines; lymphocytes; granulocytes; platelets; and erythroid cells. DC continued to express the 33D1 antigen after 4 days in culture, whereas M phi and lymphocytes did not acquire it. Quantitative and autoradiographic studies confirmed that spleen and lymph node suspensions contain less than 1% DC. We conclude that 33D1 Ab detects a stable and specific DC antigen and can be used to monitor DC content in complex lymphoid mixtures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Klinkert W. E., LaBadie J. H., O'Brien J. P., Beyer C. F., Bowers W. E. Rat dendritic cells function as accessory cells and control the production of a soluble factor required for mitogenic responses of T lymphocytes. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5414–5418. doi: 10.1073/pnas.77.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med. 1980 May 1;151(5):1196–1212. doi: 10.1084/jem.151.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Gutchinov B., Cohn Z. A. Dendritic cells are accessory cells for the development of anti-trinitrophenyl cytotoxic T lymphocytes. J Exp Med. 1980 Oct 1;152(4):1070–1084. doi: 10.1084/jem.152.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Unkeless J. C., Witmer M. D., Gutchinov B., Cohn Z. A. Studies of the cell surface of mouse dendritic cells and other leukocytes. J Exp Med. 1981 Jul 1;154(1):168–187. doi: 10.1084/jem.154.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Parker J. W., Frelinger J. A., O'Brien R. L. Characterization of responding cells in oxidative mitogen stimulation. II. Identification of an Ia-bearing adherent accessory cell. J Immunol. 1980 Jun;124(6):2700–2707. [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974 Feb 1;139(2):380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Machtinger B. G., Fried J., Cohn Z. A. Mouse spleen lymphoblasts generated in vitro. Recovery in high yield and purity after floatation in dense bovine plasma albumin solutions. J Exp Med. 1978 Feb 1;147(2):279–296. doi: 10.1084/jem.147.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Witmer M. D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. F., Dean J., Eilat D., Lorenz P. E., Schechter A. N. Tritium labeling of proteins to high specific radioactivity by reduction methylation. J Biol Chem. 1980 Sep 25;255(18):8842–8847. [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]