Abstract
This paper presents data on reactions of murine macrophages with a variety of lectins, with special focus on Griffonia simplicifolia I-B4 isolectin, the only lectin we tried that distinguishes stimulated macrophages from resident populations. Specificity of Griffonia simplicifolia I reaction with carbohydrate determinants at the cell surface is shown by (i) ability of alpha-galactosidase treatment of intact cells to abolish all lectin binding whereas beta-galactosidase has no effect on lectin binding, (ii) ability of methyl alpha-D-galactopyranoside to completely inhibit lectin binding with methyl alpha-D-galactopyranoside having no effect on lectin binding, (iii) ability of brief treatment of intact cells with trypsin to liberate a glycopeptide but reacts with G. simplicifolia I to form a precipitate that is dissolved by addition of methyl-alpha-D-galactopyranoside or alpha-galactosidase, (iv) ability of methyl alpha-D-galactopyranoside (but no other monosaccharide) to completely inhibit avid binding of macrophages to G. simplicifolia I lectin immobilized on an insoluble support, and (v) ability of immobilized lectin to separate macrophages into highly pure subpopulations of lectin-reactive and lectin-unreactive cells, as shown by examination of fluorescein-labeled lectin-treated cells with phase-contrast/fluorescence microscopy.
Full text
PDF

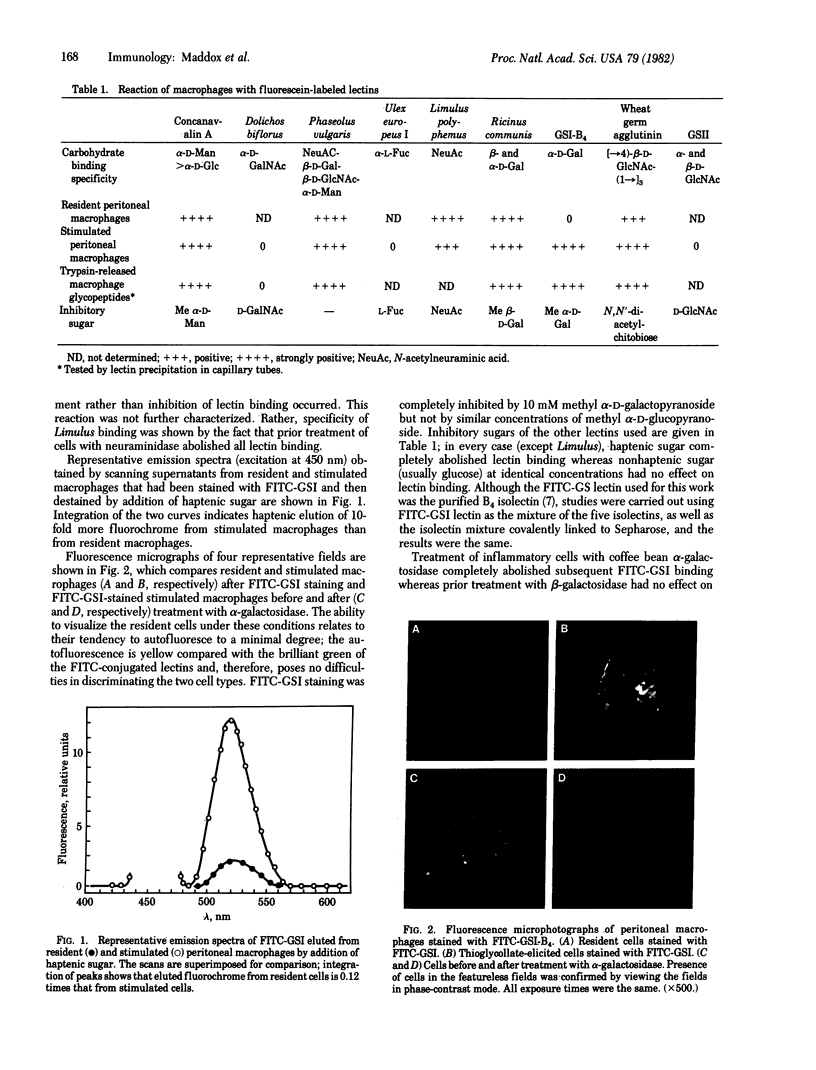
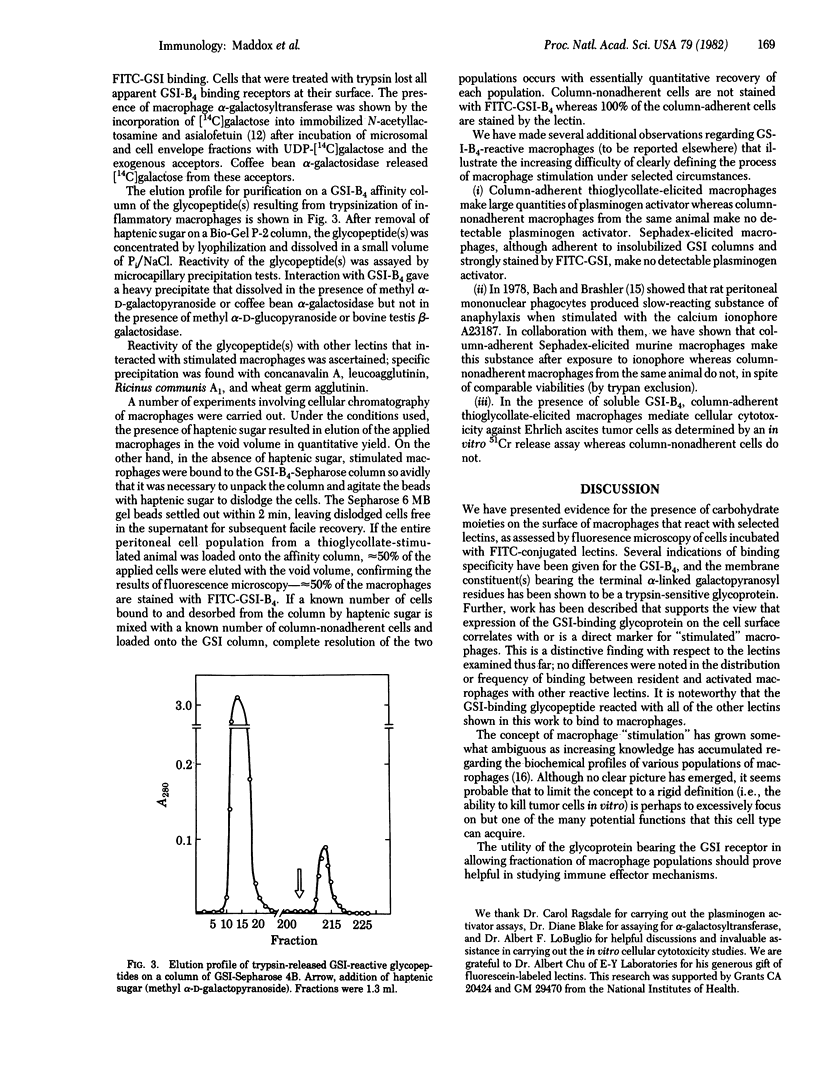

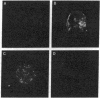
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. K., Brashler J. R. Ionophore A 23187-induced production of slow reacting substance of anaphylaxis (SRS-A) by rat peritoneal cells in vitro: evidence for production by mononuclear cells. J Immunol. 1978 Mar;120(3):998–1005. [PubMed] [Google Scholar]
- Blake D. A., Goldstein I. J. An alpha-D-galactosyltransferase activity in Ehrlich ascites tumor cells. Biosynthesis and characterization of a trisaccharide (alpha-D-galactose-(1 goes to 3)-N-acetyllactosamine). J Biol Chem. 1981 Jun 10;256(11):5387–5393. [PubMed] [Google Scholar]
- Davies P., Bonney R. J. Secretory products of mononuclear phagocytes: a brief review. J Reticuloendothel Soc. 1979 Jul;26(1):37–47. [PubMed] [Google Scholar]
- Delmotte F. M., Goldstein I. J. Improved procedures for purification of the Bandeiraea simplicifolia I isolectins and Bandeiraea simplicifolia II lectin by affinity chromatography. Eur J Biochem. 1980 Nov;112(2):219–223. doi: 10.1111/j.1432-1033.1980.tb07197.x. [DOI] [PubMed] [Google Scholar]
- Distler J. J., Jourdian G. W. The purification and properties of beta-galactosidase from bovine testes. J Biol Chem. 1973 Oct 10;248(19):6772–6780. [PubMed] [Google Scholar]
- Monsigny M., Sene C., Obrenovitch A. Quantitative fluorimetric determination of cell-surface glycoconjugates with fluorescein-substituted lectins. Eur J Biochem. 1979 May 15;96(2):295–300. doi: 10.1111/j.1432-1033.1979.tb13040.x. [DOI] [PubMed] [Google Scholar]
- Ogmundsdóttir H. M., Weir D. M. Mechanisms of macrophage activation. Clin Exp Immunol. 1980 May;40(2):223–234. [PMC free article] [PubMed] [Google Scholar]
- Peters B. P., Goldstein I. J. The use of fluorescein-conjugated Bandeiraea simplicifolia B4-isolectin as a histochemical reagent for the detection of alpha-D-galactopyranosyl groups. Their occurrence in basement membranes. Exp Cell Res. 1979 May;120(2):321–334. doi: 10.1016/0014-4827(79)90392-6. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Barcinski M. A., Rosenwasser L. J. Function of macrophages in genetic control of immune responsiveness. Fed Proc. 1978 Jan;37(1):79–85. [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Cooperation between mononuclear phagocytes and lymphocytes in immunity. N Engl J Med. 1980 Oct 23;303(17):977–985. doi: 10.1056/NEJM198010233031706. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- WALLACH D. F., KAMAT V. B. PLASMA AND CYTOPLASMIC MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA. Proc Natl Acad Sci U S A. 1964 Sep;52:721–728. doi: 10.1073/pnas.52.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Douglas S. D. Dynamics of the macrophage plasma membrane. Annu Rev Microbiol. 1979;33:267–307. doi: 10.1146/annurev.mi.33.100179.001411. [DOI] [PubMed] [Google Scholar]