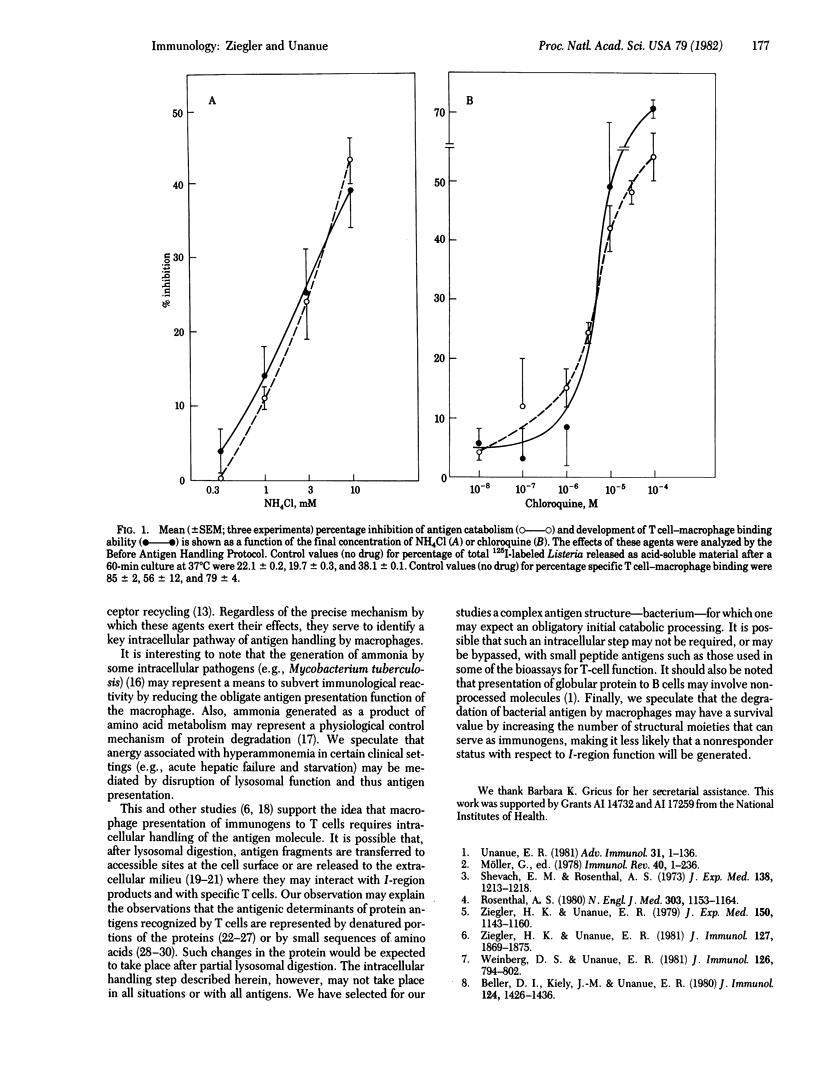
Abstract
This paper describes the effects of two lysosomotropic compounds, ammonia and chloroquine, on the interaction of mononuclear phagocytes with immune T cells. The uptake and ingestion of Listeria monocytogenes by macrophages were not affected by the drugs; however, the macrophage catabolism of 125I-labeled Listeria was reduced in a dose-dependent way. The macrophage presentation of Listeria to T cells, an I-region-dependent phenomenon, was also inhibited. The degree of inhibition of catabolism paralleled that of antigen presentation. The inhibition of antigen presentation took place if the macrophages were treated before and during Listeria uptake; minimal inhibition took place if the macrophages were treated 30 min after Listeria ingestion, at which time a significant amount of bacteria was already catabolized. Our previous studies had shown that the macrophage-associated antigen recognized by T cells became apparent 30-60 min after uptake of Listeria. We conclude that ammonia and chloroquine affected an intracellular handling step required for the expression of the immunogen relevant for T-cell recognition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I., Kiely J. M., Unanue E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunologic stimuli. J Immunol. 1980 Mar;124(3):1426–1432. [PubMed] [Google Scholar]
- Calderon J., Unanue E. R. The release of antigen molecules from macrophages: characterization of the phenomena. J Immunol. 1974 May;112(5):1804–1814. [PubMed] [Google Scholar]
- Chesnut R. W., Endres R. O., Grey H. M. Antigen recognition by T cells and B cells: recognition of cross-reactivity between native and denatured forms of globular antigens. Clin Immunol Immunopathol. 1980 Mar;15(3):397–408. doi: 10.1016/0090-1229(80)90051-3. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Lipsky P. E., Rosenthal A. S. Antigen handling by guinea pig macrophages: further evidence for the sequestration of antigen relevant for activation of primed T lymphocytes. J Immunol. 1977 Jun;118(6):2053–2057. [PubMed] [Google Scholar]
- Erb P., Meier B., Feldmann M. Is genetically related macrophage factor (GRF) a soluble immune response (Ir) gene product? J Immunol. 1979 May;122(5):1916–1919. [PubMed] [Google Scholar]
- Farr A. G., Kiely J. M., Unanue E. R. Macrophage-T cell interactions involving Listeria monocytogenes--role of the H-2 gene complex. J Immunol. 1979 Jun;122(6):2395–2404. [PubMed] [Google Scholar]
- Farr A. G., Wechter W. J., Kiely J. M., Unanue E. R. Induction of cytocidal macrophages after in vitro interactions between Listeria-immune T cells and macrophages--role of H-2. J Immunol. 1979 Jun;122(6):2405–2412. [PubMed] [Google Scholar]
- GELL P. G., BENACERRAF B. Studies on hypersensitivity. II. Delayed hypersensitivity to denatured proteins in guinea pigs. Immunology. 1959 Jan;2(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- Geisow M. J., D'Arcy Hart P., Young M. R. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J Cell Biol. 1981 Jun;89(3):645–652. doi: 10.1083/jcb.89.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer H., Heinrich P. C. Control of proteolysis. Annu Rev Biochem. 1980;49:63–91. doi: 10.1146/annurev.bi.49.070180.000431. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Kishimoto T., Delespesse G., King T. P. Immunogenic properties of modified antigen E. I. Presence of specific determinants for T cells in denatured antigen and polypeptide chains. J Immunol. 1974 Jul;113(1):70–77. [PubMed] [Google Scholar]
- Lonai P., Puri J., Hämmerling G. H-2-restricted antigen binding by a hybridoma clone that produces antigen-specific helper factor. Proc Natl Acad Sci U S A. 1981 Jan;78(1):549–553. doi: 10.1073/pnas.78.1.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C. R., Liew F. Y. Immune response to chemically modified flagellin. 3. Enhanced cell-mediated immunity during high and low zone antibody tolerance to flagellin. J Exp Med. 1972 Feb 1;135(2):298–311. doi: 10.1084/jem.135.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S. Regulation of the immune response--role of the macrophage. N Engl J Med. 1980 Nov 13;303(20):1153–1156. doi: 10.1056/NEJM198011133032005. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V., Wigzell H. Immune responses against native and chemically modified albumins in mice. II. Effect of electric charge and conformation on the humoral antibody response and on helper T cell responses. J Immunol. 1974 Nov;113(5):1635–1643. [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Rosenthal A. S. Function of macrophages in antigen recognition by guinea pig T lymphocytes. II. Role of the macrophage in the regulation of genetic control of the immune response. J Exp Med. 1973 Nov 1;138(5):1213–1229. doi: 10.1084/jem.138.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Meltz S. K., Wilner G. D. Nature of T lymphocyte recognition of macrophage-associated antigens. I. Response of guinea pig T cells to human fibrinopeptide B. J Immunol. 1979 Aug;123(2):759–764. [PubMed] [Google Scholar]
- Thomas D. W., Meltz S. K., Wilner G. D. Nature of T lymphocyte recognition of macrophage-associated antigens. II. Macrophage determination of guinea pig T cell responses to human fibrinopeptide B. J Immunol. 1979 Sep;123(3):1299–1302. [PubMed] [Google Scholar]
- Thompson K., Harris M., Benjamini E., Mitchell G., Noble M. Cellular and humoral immunity: a distinction in antigenic recognition. Nat New Biol. 1972 Jul 5;238(79):20–21. doi: 10.1038/newbio238020a0. [DOI] [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Chloroquine and ammonium ion inhibit receptor-mediated endocytosis of mannose-glycoconjugates by macrophages: apparent inhibition of receptor recycling. Biochem Biophys Res Commun. 1980 Mar 13;93(1):1–8. doi: 10.1016/s0006-291x(80)80237-3. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Weinberg D. S., Unanue E. R. Antigen-presenting function of alveolar macrophages: uptake and presentation of Listeria monocytogenes. J Immunol. 1981 Feb;126(2):794–799. [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. The specific binding of Listeria monocytogenes-immune T lymphocytes to macrophages. I. Quantitation and role of H-2 gene products. J Exp Med. 1979 Nov 1;150(5):1143–1160. doi: 10.1084/jem.150.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]



