Abstract
We have found that, in chylomicrons from intestinal lymph fistulas in the rat, the sole molecular species of apolipoprotein B (apo B) is B-48. This protein is analogous to the B-48 apoprotein of human chylomicrons. In contrast, preparations of chylomicrons from blood serum are known to contain species of B apolipoproteins of higher molecular weight, presumably due to the presence of hepatogenous lipoproteins. We studied the removal of 125I-labeled apo B-48 of intestinal lymph chylomicrons from blood plasma of rats. The removal of [14C]cholesteryl esters of biologically labeled chylomicrons was unaffected by radioiodination. The labeled cholesteryl esters and apo B-48 disappeared rapidly from the density (P) less than 1.006 g/ml fraction of plasma. In contrast to the apo B of very low density lipoproteins (1.019 less then p less then 1.063 g/ml) after 1 hr. No 125I was found in the B-100 or B-95 apolipoproteins at any time. We conclude that, unlike the species of apo B found uniquely in hepatogenous very low density lipoproteins, the apo B-48 protein of chylomicrons is not a precursor of the B apoprotein of low density lipoproteins.
Full text
PDF
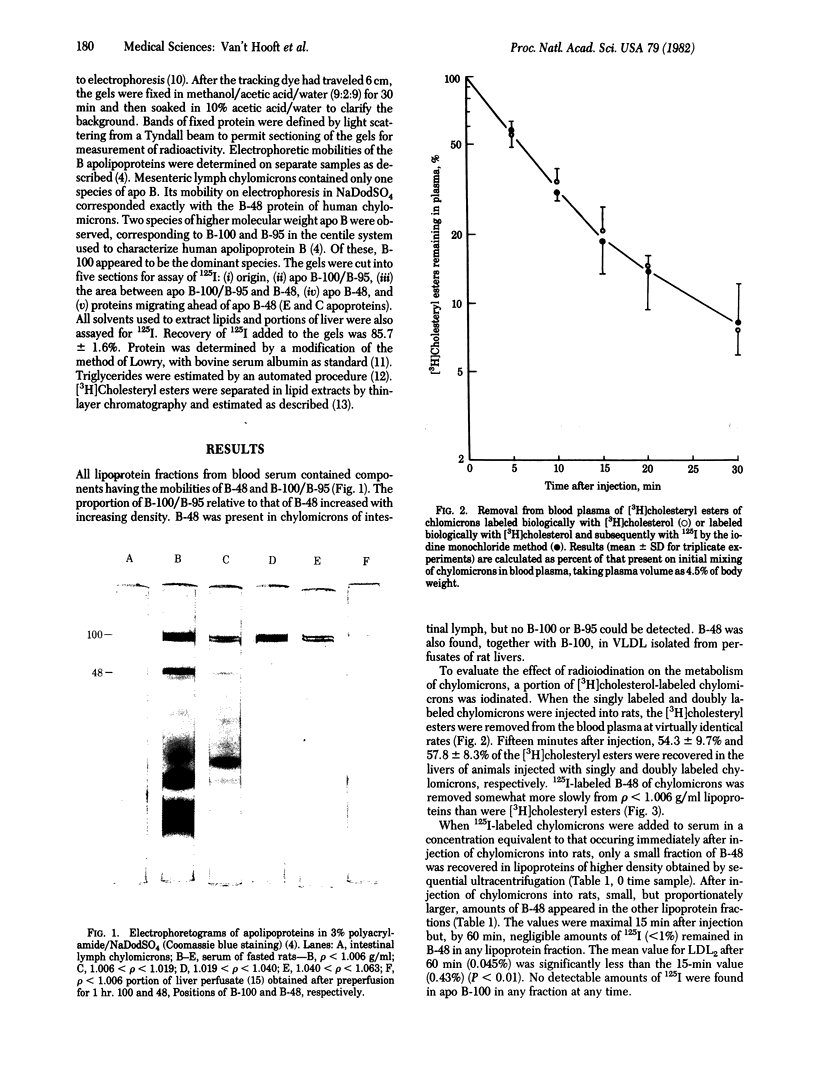
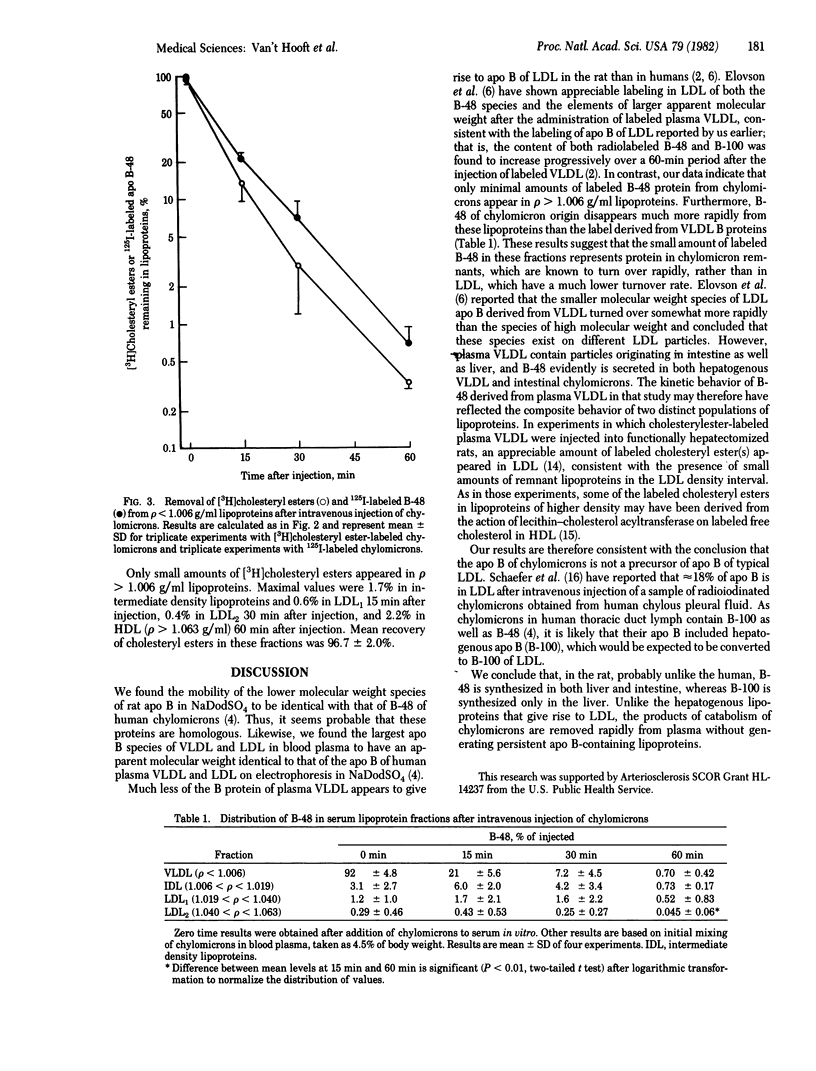

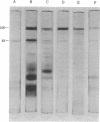
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elovson J., Huang Y. O., Baker N., Kannan R. Apolipoprotein B is structurally and metabolically heterogeneous in the rat. Proc Natl Acad Sci U S A. 1981 Jan;78(1):157–161. doi: 10.1073/pnas.78.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faergeman O., Sata T., Kane J. P., Havel R. J. Metabolism of apoprotein B of plasma very low density lipoproteins in the rat. J Clin Invest. 1975 Dec;56(6):1396–1403. doi: 10.1172/JCI108220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J. Validation of a procedure for exogenous isotopic labeling of lipoprotein triglyceride with radioactive triolein. Biochim Biophys Acta. 1979 May 25;573(2):255–265. doi: 10.1016/0005-2760(79)90059-6. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Williams M. C., Fielding C. J., Havel R. J. Discoidal bilayer structure of nascent high density lipoproteins from perfused rat liver. J Clin Invest. 1976 Sep;58(3):667–680. doi: 10.1172/JCI108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman D. A., Kane J. P. Improved separation of high-molecular-weight proteins by preparative sodium dodecyl sulfate--gel electrophoresis: application to apolipoprotein B. Anal Biochem. 1980 Jun;105(1):174–180. doi: 10.1016/0003-2697(80)90442-x. [DOI] [PubMed] [Google Scholar]
- Imaizumi K., Fainaru M., Havel R. J. Composition of proteins of mesenteric lymph chylomicrons in the rat and alterations produced upon exposure of chylomicrons to blood serum and serum proteins. J Lipid Res. 1978 Aug;19(6):712–722. [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. P., Sata T., Hamilton R. L., Havel R. J. Apoprotein composition of very low density lipoproteins of human serum. J Clin Invest. 1975 Dec;56(6):1622–1634. doi: 10.1172/JCI108245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaiah K. V., Walker L. F., Borensztajn J., Schonfeld G., Getz G. S. Apolipoprotein B variant derived from rat intestine. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3806–3810. doi: 10.1073/pnas.77.7.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy M. J., Kane J. P., Hardman D. A., Hamilton R. L., Dalal K. B. Normotriglyceridemic abetalipoproteinemia. absence of the B-100 apolipoprotein. J Clin Invest. 1981 May;67(5):1441–1450. doi: 10.1172/JCI110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Mjos O. D., Faergeman O., Hamilton R. L., Havel R. J. Characterization of remnants produced during the metabolism of triglyceride-rich lipoproteins of blood plasma and intestinal lymph in the rat. J Clin Invest. 1975 Sep;56(3):603–615. doi: 10.1172/JCI108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer E. J., Jenkins L. L., Brewer H. B., Jr Human chylomicron apolipoprotein metabolism. Biochem Biophys Res Commun. 1978 Jan 30;80(2):405–412. doi: 10.1016/0006-291x(78)90691-5. [DOI] [PubMed] [Google Scholar]
- Sigurdsson G., Nicoll A., Lewis B. Conversion of very low density lipoprotein to low density lipoprotein. A metabolic study of apolipoprotein B kinetics in human subjects. J Clin Invest. 1975 Dec;56(6):1481–1490. doi: 10.1172/JCI108229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R. J. Determinants of hepatic uptake of triglyceride-rich lipoproteins and their remnants in the rat. J Biol Chem. 1980 Jun 10;255(11):5475–5480. [PubMed] [Google Scholar]