Abstract
Background
Despite widespread use of chronic intrathecal (IT) infusions of morphine, there is little systematic human work evaluating the steady-state morphine concentrations or cerebrospinal (CSF) chemistry after long-term IT morphine delivery. We sought to address these issues in patients receiving chronic IT morphine infusion.
Methods
Pain patients with implanted catheters and pumps (range: 127–2165 days), receiving a stable dosing (> 1 week) of IT morphine by infusion, were entered into the study. The following sequence was performed: 1) estimation of pain score; 2) radiograph localization of catheter tip; 3) Percutaneous sampling of lumbar CSF at the L4-5 or L5-S1 space. CSF/plasma samples were assayed for chemistry, and morphine and its 3/6 glucuronide metabolites (M3G, M6G) by liquid chromatography mass spectrometry.
Results
Nineteen patients were enrolled. CSF samples were obtained from 16 subjects. Three patients were not included in the primary analysis because one catheter was epidural, one catheter was fractured and one had a granuloma at the catheter tip. Of the 13 sampled patients, the range of daily doses, rates and concentrations were 1.6–25 mg/d and 0.1–1 ml/d, 5–50 mg/mL, respectively. The principal observations were: i) morphine, M3G and M6G were present in the CSF and plasma and showed a significant regression slope when plotted versus daily dose; ii) in contrast, the regression slope of the group ratio Morphine: M3G: M6G plotted versus daily dose in CSF or plasma was not different from zero; iii) plotting “normalized” CSF analyte concentration (e.g., concentration at site/daily IT morphine dose) against the segmental distance of the sampling site from the catheter tip revealed a significant decline in concentration of morphine, but not of conjugates as a function of distance from the catheter tip; iv) plotting CSF protein, glucose, red and white cell counts versus daily morphine dose or morphine concentration at the sampling site revealed no significant regression; and v) patients with a catheter failure or a granuloma showed reduced concentrations of morphine in their CSF.
Conclusion
Chronic infusion of morphine shows high concentrations which correlate with the infusion dose and the proximity of the sampling site to the infusion site with no effects on CSF chemistry.
Introduction
The spinal delivery of morphine results in a clinically effective analgesia through activation of spinal opioid receptors.1 This observation led to the delivery of morphine via an implanted infusion pump for a variety of clinical pain states.2–8 Despite widespread use, the steady-state morphine (MOR) and MOR metabolite concentrations in the cerebrospinal fluid (CSF) and peripheral circulation, or the effects upon CSF chemistry (protein, glucose, white blood cells [WBC]) after long-term spinal infusion have not been systematically studied. Previous human kinetic work has largely focused on CSF and plasma concentrations after acute bolus delivery.9–11 More information on chronic delivery is needed to better understand the potential effects of MOR on spinal tissues, such as the granuloma which has been argued to result from high local concentrations.12,13
An additional variable of importance relates to the rostrocaudal distribution of drug from the drug infusion site. The overall concentrations of MOR in the plasma after intrathecal delivery (reflecting MOR which is cleared from the intrathecal space) is proportional to the daily infusion dose. An important variable for the CSF concentrations is the intrathecal sampling site relative to the infusion site. There is ample porcine work which suggests after the intrathecal infusion of drugs such as MOR there is a steep rostrocaudal concentration gradient as one samples at increasing distances from the catheter tip.14–16 Though this is intuitively relevant, such a gradient has not been systematically examined in humans under the steady-state conditions as achieved by continuous infusion. The presence of this gradient is believed to reflect the absence of a robust CSF flow and the role of local dilution and diffusion of the injectate from the tip where the infusion volume is small relative to the local CSF volume.15,17 Such a concentration gradient away from the infusion site has theoretical and practical relevance. First, we believe the ability of an intrathecal opiate to produce analgesia depends upon the ability of the infusate to achieve adequate concentrations of drug over a length of spinal cord corresponding to the rostrocaudal distribution of the spinal afferent terminals of the dermatomes to be blocked. Second, if intrathecal MOR indeed has an effect upon the clinical chemistry, we hypothesized that the effect would be concentration dependent and the magnitude of the effects upon CSF chemistry should co-vary with the local concentrations of MOR at the sampling sites, as opposed to the absolute daily dose that the patient received. Third, the presence of morphine 3 glucuronide (M3G) and morphine 6 glucuronide (M6G) has been demonstrated in CSF after intrathecal delivery.9–11,18 An important question is whether these metabolites arise from conversion of intrathecal MOR in brain neural tissues19 or do they represent MOR which has moved into the peripheral circulation where it undergoes conjugation and then a central redistribution?20 If the metabolites derive from spinal tissue, we hypothesize that they will show a gradient concentration reduction from the catheter tip similar to what is observed with MOR. If redistributed from the periphery, there will be no gradient. Finally, given that high concentrations of opiates can evoke changes in local cord pathology, there is concern whether such infusion might have an effect upon local clinical chemistry that is related to the drug concentration at the sampling site.
To specifically address the above issues in patients receiving stable infusions of intrathecal MOR for an interval of at least 7 days we examined 1) the effect of MOR dose on concentration of MOR and its metabolites, M3G and M6G, in CSF and plasma; 2) the effect of sampling site on local concentrations of MOR and its metabolites, and 3) the relationship between daily infusion dose, local CSF MOR concentration and lumbar CSF clinical chemistry.
Methods
Patient Population
This study was approved by the University of California, San Diego IRB. Patients who were receiving intrathecal MOR alone or MOR + clonidine (one subject) through a chronically implanted catheter and had no changes in the delivered dose for at least one week were eligible for the study. If the patients were receiving systemic MOR, they were changed to an equivalent dose of a non-MOR opioid (i.e., oxycodone, methadone, fentanyl patch) and allowed a 1-week washout of systemic MOR. After informed written consent, the following was obtained from the subject: 1) average pain score over the previous week using the visual analog scale (VAS); 2) radiograph localization of the catheter tip; 3) ability to withdraw CSF from the side port; 4) if there was a positive aspiration of CSF from the side port, the catheter contents were removed and the catheter integrity was assessed by injecting contrast through the side port under fluoroscopy; and 5) lumbar CSF was typically sampled at the L4-5 or L5-S1 space (Percutaneous; 22 gauge; 4 mL). For presentation, vertebral levels from the thoracic to the sacral are numbered sequentially. Thus, T12 = 12, L3 = 15, etc.
Sample assays
Samples were assayed for: chemistry (cell count with differential, electrolytes, protein, glucose) (1 mL); gram stain; and MOR, M3G, and M6G (2 mL). Plasma samples (5 mLs) were assayed for complete blood count with differential; complete chemistry panel including liver function (1 mL); MOR, M3G and M6G (2 mL).
Assay Techniques
Samples were assayed after extraction by liquid chromatograph–mass spectrometry using a method similar to that previously published.21 In brief, a solid-phase extraction served to isolate MOR, M3G, M6G and their deuterated internal standards from CSF and plasma. The extract was delivered onto a liquid chromatograph tandem mass spectrometer. Chromatographic separation of the three peaks was achieved on a silica column with an aqueous organic mobile phase. Assay and extraction techniques had been validated for human plasma and CSF, with the standard curve range in plasma being approximately 0.5–50 ng/ml for MOR, 1.0–100 ng/ml for M6G and 10–1000 ng/ml for M6G. Samples below the assay detection limits of the standard curve were not included in calculations or data plotting.
Analysis of Hypotheses
Because these patients were clinical patients, no two patients received the same infusion dose. However, we can assume that diffusion of drug away from the catheter tip is a first-order process (i.e., rate is independent of dose). Each measured CSF concentration was divided by the concurrent daily infusion dose to produce a “normalized” CSF concentration. The relationship between the distance from catheter tip to sampling site (independent variable) was then plotted against the normalized CSF opioid concentration (dependent variable). Linear regression of these data was used to test the hypothesis that CSF concentration varies with the distance from the catheter tip (slope of the regression line is significantly different from zero). Statistical analyses were performed using Prism v.4 for MacIntosh (GraphPad Prism Software Inc., San Diego, CA). Differences or regression slopes reaching a level of p < 0.05 were counted as being statistically significant.
Results
Patient Population
Nineteen patients were enrolled (6 males, 13 females). Sixteen patients were successfully sampled and assayed for CSF MOR and metabolites. Three of these patients, #4, #6 and #7 are not included in the primary analysis and are discussed separately below. Patient #4 had a dysfunctional catheter that was believed to be epidural. Patient #7 had a fractured catheter that was later discovered when she had a pump reimplant. On magnetic resonance imaging (MRI), patient #6 displayed a granuloma at the catheter tip. The characteristics of the included patient population are presented in Table 1. One patient also received clonidine (1500 mcg/ml) as an adjuvant. Inspection of data did not reveal any CSF concentrations or CSF chemistry that would be considered an outlier and these data were included in the analysis.
TABLE 1.
Patient Population Variables
| VARIABLE | MALE | FEMALE | GROUP |
|---|---|---|---|
| Number | 6 | 8a | 13 |
| Age (yrs) | 54± 2 (48 – 62) | 60 ± 6 (40 –76) | 57 ± 3 (40 –76) |
| Weight (lbs) | 186 ±16 (146–235) | 136± 7 (98 – 165) | 158 ± 10 (98 – 235) |
| Height (in) | 69 ± 1 (67 – 72) | 62 ± 1 ( 58–66) | 65 ± 1 ( 58–72) |
| Duration of implant (days) | 932 ± 129 ( 399–1364) | 1162 ± 211 (127 – 2165) | 1063 ± 132 (127 – 2165) |
| Location of catheter tipb | 12 ± 1 (9–15) | 10.8 ± 0.3 (9.5– 12) | 11 ±1 (8.5– 14.5) |
| Daily Morphine Infusion Dose (mg) | 7.9 ± 2.8 (2 – 20.5) | 12.6 ± 2.8 (1.6–25) | 10.6 ± 2.0 (1.6–25) |
| Morphine Conc. (mg/ml) | 31 ±8.0 (5 – 50) | 32±6 (5–50) | 31±5 (5–50) |
| Flow rate (ml/d) | 0.29 ±0.05 (0.1–0.4) | 0.41±0.09 (0.24–1.0) | 0.36±0.06 (0.1–1.0) |
| Pain score | 62±10 (18–82) | 61±10 (0–89) | 61±7 (0–89) |
1 female is included, but has no cerebrospinal fluid (CSF) drug assays.
For presentation, vertebral levels from the thoracic to the sacral are numbered sequentially 9–15. Thus, T8 = 8, L3 = 15. Ranges are given in ( ).
Morphine and Metabolites
MOR, M3-glucuronide, and M6-glucuronide concentrations were assayed in the CSF and plasma. All three molecules were measurable in CSF and plasma. The respective concentrations showed a significant slope for the regression line plotting analyte versus dose in both CSF and plasma (Figures 1 and 2).
Figure 1.
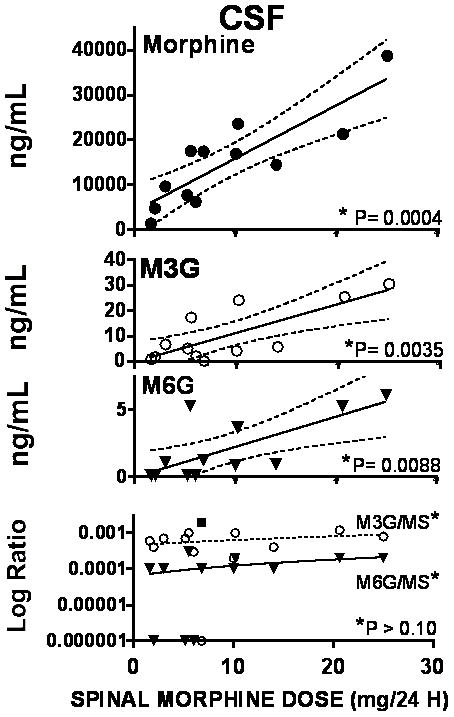
Graphs plot the best fit regression line for the cerebrospinal fluid (CSF) concentrations of morphine, morphine 3 glucuronide (M3G) and morphine 6 glucuronide (M6G) plotted as a function of the daily intrathecal dose of morphine. Bottom graph plots the ratio of M3G to morphine (MS) and M6G to morphine in CSF as a function of daily dose. The ratio of M6G to M3G was calculated (not shown) * in each graph indicates the statistical significance of the slope of the regression lines of the respective plot. As shown, the slope of the regression lines for morphine, M3G and M6G were statistically significant while the slope of the regression lines for the ratio versus dose was not. Where a statistically significant slope was found, the 95% CI is plotted (dashed lines)
Figure 2.
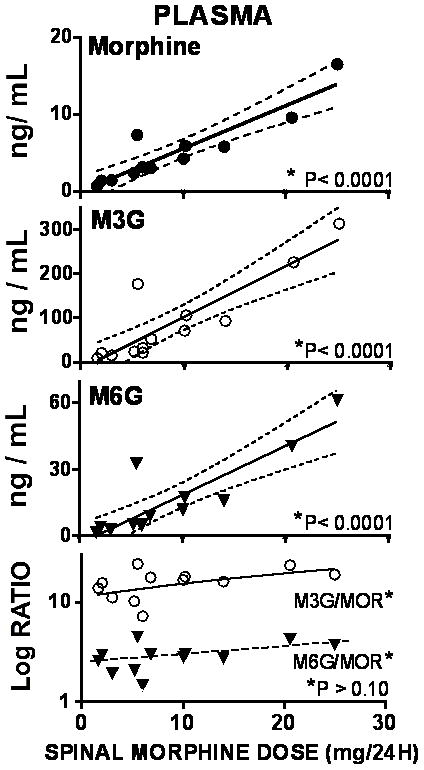
Graphs plot the best fit regression line for the plasma concentrations of morphine, morphine 3 glucuronide (M3G) and morphine 6 glucuronide (M6G) plotted as a function of the daily intrathecal dose of morphine. Bottom graph plots the ratio of M3G to morphine (MS) and M6G to morphine in cerebrospinal fluid (CSF) as a function of daily dose. The ratio of M6G to M3G was calculated (not shown) * in each graph indicates the statistical significance of the slope of the regression lines of the respective plot. As shown, the slopes of the regression lines for morphine, M3G and M6G were statistically significant, while the slope of the regression lines for the ratio versus dose was not. Where a statistically significant slope was found, the 95% CI is plotted (dashed lines)
Because there was no significant regression of analyte ratios of M3G/MOR and M6G/MOR and M6G/M3G) in CSF and plasma versus daily dose (Figures 1 and 2), mean ratios in CSF and plasma were calculated. As indicated in Table 2, metabolite ratios showed higher concentrations of metabolite than MOR in plasma while the converse was true in CSF. Conversely, the M3G/M6G ratios were comparable in both CSF and plasma.
Table 2.
Morphine metabolite ratios in cerebrospinal fluid (CSF) and plasma.*
| SOURCE | GROUP | ||
|---|---|---|---|
| M3G/MOR | M6G/MOR | M6G/M3G | |
| CSF | 0.00058 ± 0.0011 | 0.00011 ± 0.00003 | 0.159 ± 0.038 |
| PLASMA | 13.9 ± 1.68 | 2.48 ± 0.30 | 0.16 ± 0.04 |
Mean ± SEM of all patients.
MOR = morphine
M3G = morphine 3 glucuronide
M6G = morphine 6 glucuronide
Plotting the CSF to plasma ratio for MOR, M3G and M6G versus daily spinal MOR infusion reveals that there is no significant slope of the regression of metabolite on daily dose, indicating that the relative distribution of each drug is independent of the infusion dose over the range of doses used. (Figure 3) The group mean (±SEM) CSF to plasma ratio for MOR, M3G and M6G were 3111±353, 0.15±0.04, and 0.16±0.04 respectively.
Figure 3.
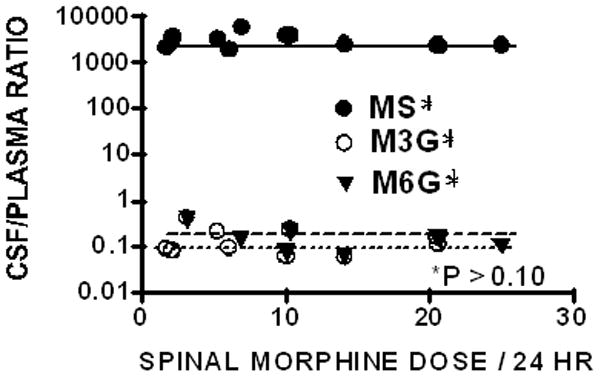
CSF to plasma ratio for morphine (MS), morphine 3 glucuronide (M3G) or morphine 6 glucuronide (M6G) are plotted versus daily Infusion dose of morphine. No regression slope reached statistical significance.
Effects of Patient Variables on CSF Morphine
We sought to consider the contributions of patient characteristics and sampling site on the local concentrations of MOR in the CSF.
Patient Characteristics
To assess the contribution of patients’ variables to lumbar CSF concentrations over dose, we examined the regression of normalized lumbar CSF concentrations (measured CSF concentration of molecule divided by the daily infusion dose, e.g., MOR/24 hrs infusion dose) on i) age, ii) body weight, and iii) height. In no case was there a significant regression of this ratio upon the variable (p > 0.10; data not shown). Means of groups comparing males and females with a simple t-test showed no significant differences (p > 0.10; data not shown).
Examination of the VAS scores revealed no significant covariance with either total daily MOR infusion dose or with lumbar CSF concentrations of MOR (p > 0.10, data not shown). Mean (± SEM) and median (25th/75th percentile) VAS scores in this population were 63 (± 6) and 73 (54, 80), respectively.
Sampling Site
Catheter tips were identified to range from T8 to L2. To assess the distribution of drug from the catheter tip, we plotted the CSF MOR concentration, normalized by dividing by the daily dose, against the segmental distance of the sampling catheter from the tip. As indicated in Figure 4, there was a clear and statistically significant regression of the normalized MOR concentration in the CSF ratio when plotted against catheter sampling site, the slope of which was statistically significant for MOR, but not the metabolites. In contrast, plotting the plasma dose ratio revealed no change over segmental concentrations for MOR or either metabolite (Figure 4). This lack of a regression of plasma MOR emphasizes that no matter where the infusion site, the plasma concentrations are related to infusion dose. This further makes the point that MOR clearance into the plasma was similar for all patients no matter the infusion dose.
Figure 4.
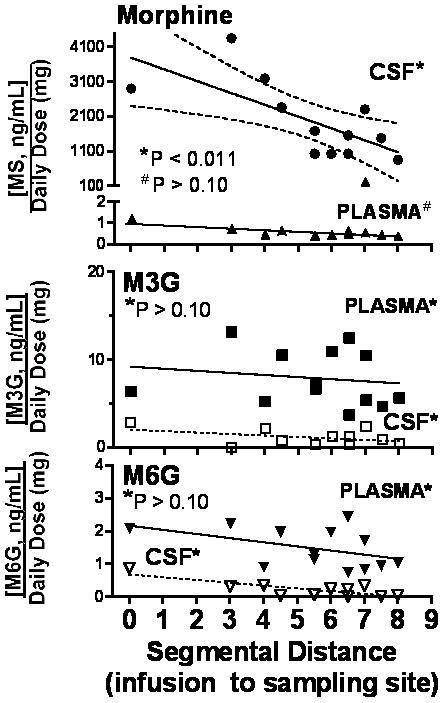
Normalized concentrations of morphine (top), morphine 3 glucuronide (M3G) or morphine 6 glucuronide (M6G) in cerebrospinal fluid (CSF) and plasma (e.g. measured CSF concentrations/daily infusion dose of morphine) are plotted as a function of the segmental distance (number of spinal segments) between the sampling site and the infusion site. * in each graph indicates the statistical significance of the slope of the regression lines of the respective plot. As shown, only the slope of the regression line for CSF morphine, was statistically significant, Where a statistically significant slope was found, the 95% CI is plotted (dashed lines)
CSF Clinical Chemistry
The regression analysis of counts (WBC and red blood cell [RBC]) or concentration (protein and glucose) versus daily MOR dose (Figure 5) or versus measured MOR concentration (not shown) indicated no statistical significance. Mean protein, glucose, RBC and WBC levels (+SEM)(ranges) were 56+13 mg/dL (25–253), 71+5 mg/dL (54–1270), 1187+636 (0–7000), and 4+1 (0–2) respectively. As indicated, glucose was normal. CSF protein and WBC count were slightly increased over normal. These values are likely explained by bloody taps in 3 subjects resulting in abnormally high WBC counts. Gram stains were uniformly negative (data not shown).
Figure 5.
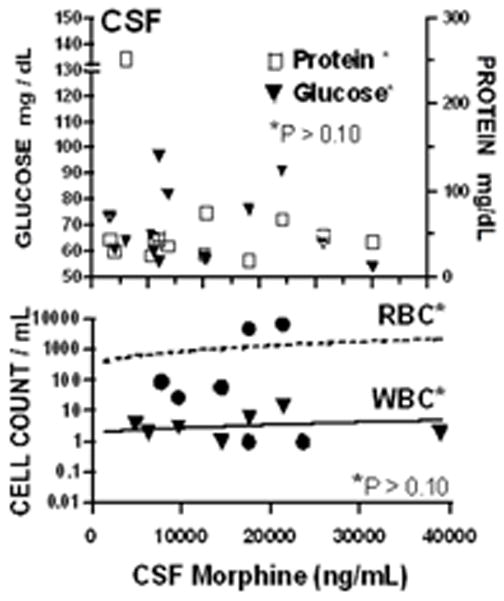
Concentrations of protein and glucose (top) or red and white blood cell counts (bottom) in cerebrospinal fluid (CSF) are plotted as a function of daily intrathecal dose of morphine. No regression slope was statistically significant over dose (p > 0.10).
Patient Outliers
Patients #4, #6 and #7 are not included in the above analysis and are discussed separately. Patient #4 had a dysfunctional catheter that was believed to be epidural. Patient #7 had a fractured catheter that was later discovered when she had a pump reimplant. On MRI, patient #6 displayed a granuloma at the catheter tip. Table 3 summarizes the variables characterizing these three patients. For comparison, the values expected based on the intrathecal dose infused in that patient and the regression relationship provided in the figures is presented. Of note, CSF MOR concentrations in the patient with an epidural catheter (#4) and the patient with a fractured catheter (#7) were well below those anticipated based on an intrathecal infusion of that dose. Interestingly, low concentrations were also noted in the granuloma patient. Plasma MOR concentrations for the granuloma patient and fractured catheter were in the expected range associated with the respective infusion doses. However, the epidural catheter was considerably higher. Considering the CSF chemistry, the patients with epidural catheter and fractured catheter showed unremarkable CSF whereas the granuloma patient displayed relatively increased protein, CSF and RBC.
TABLE 3.
Patients with Infusion Anomalies
| VARIABLE | Group Mediansa | Patient 4 Female | Patient 6 Female | Patient 7 Female |
|---|---|---|---|---|
| Diagnosis | Epidural catheter | Granuloma at catheter tip | Fractured catheter (not IT/EPI) | |
| Age (yrs) | 57 | 54 | 49 | 46 |
| Weight (lbs) | 158 | 190 | 192 | 210 |
| Height (in) | 65 | 68 | 66 | 63 |
| Duration of implant (days) | 1063 | 1420 | 742 | 461 |
| Location of catheter tip | 11 | 9.5 | 10 | 13.5 |
| Side port aspiration | + in 11/14 | Negative | Positive | Negative |
| Sampling Site | 17.5 | 16.5 | 17.5 | 17.5 |
| Myelogram | Free flow 8/8 | Not done | Normal | Not done |
| Pain scores | 61 | 62 | 80 | 90 |
| CSF Chemistry | ||||
| WBC | 4 | 4 | 20 | 0 |
| RBC | 1187 | 117 | 7000 | 2 |
| Glucose (CSF/PL) | 71/110 | 62/91 | 127/215 | 61/95 |
| Protein | 56 | 43 | 41 | 50 |
| Daily Morphine Infusion Dose (mg) | 8.4 | 1 | 36 | 17.2 |
| Drug Conc. (mg/ml) | 28 | 10 | 50 | 25 |
| CSF Morphine (ng/mL) | 15,620 | 174 (6050)b | 455 (40,100) b | 5 (20,100) b |
| Plasma Morphine (ng/mL) | 4.21 | 181 (0.6) b | 15 (20) b | 7 (9) b |
| CSF M3G (ng/mL) | 5.34 | 393 (3) b | 1319 (35) b | -- c (19) b |
| CSF M6G (ng/mL) | 1.01 | 87 (1) b | 2 (6) b | - c (4) b |
Data summarized for comparison from preceding analysis of clinical chemistries (N = 14 patients) and cerebrospinal fluid (CSF) morphine (N = 13).
Value in parentheses indicates value expected based on regression lines obtained using patient;
Below detection limit
IT = intrathecal
EPI = epidural
WBC = white blood cell
RBC = red blood cell
PL = plasma
Discussion
This clinical CSF sampling study presents an assessment of the concentrations of MOR and its metabolites in CSF and plasma in patients receiving chronic intrathecal infusion of MOR. Excluding the three dysfunctional intrathecal catheters, the data show the presence of CSF MOR concentrations with gradient that correlates with the infusion dose. It also demonstrates the presence of the conjugated metabolites with no prominent effects on CSF chemistry. The significance of these observations will be considered below.
Effects of Chronic Spinal Drug Delivery on CSF Chemistry
Despite the widespread use of spinal drug delivery for the treatment of pain, there is very little in the literature on the effects of this technique on CSF chemistry. One would predict that the presence of the foreign catheter and the drug would lead to a chronic inflammatory state that would be reflected in changes in the CSF chemistry. However, as observed in our study, the CSF glucose was within normal limits and the CSF protein was slightly increased. There was one subject with a protein of 253 mg/dL and if eliminated, the mean total protein would be within normal limits. In addition, the CSF/plasma glucose ratio was within the normal limits of 0.6.22 These studies suggest that over the range of daily exposure to the concentrations of MOR reported here, there were no proinflammatory reactions. Most spinal toxicity studies have been performed in the canine species with varying reports on the effects on CSF chemistry. Chronic infusions of intrathecal MOR, sufentanil, alfentanil and adenosine in a dog model demonstrated an increased total protein and WBCs but no effect on total glucose in both saline and drug groups.12,23,24 Chronic infusions of intrathecal neostigmine in a dog model demonstrated increases in both protein and glucose 25 whereas the infusion of clonidine and baclofen showed no effect on CSF chemistry.26,27 From these studies one cannot conclude whether the drug or catheter results in CSF chemistry changes. In the present study, there was no correlation between metabolite concentrations and CSF chemistry, whether compared as a function of total daily dose or the actual MOR concentrations assessed in the respective sample. Excluding the patient with a granuloma, two patients showed increased WBC counts. However, these two patients had a bloody tap explaining this increase and represent a false positive unrelated to the presence of spinal drug delivery. Even by including these two subjects, the mean WBC was within normal limits.
Infection is a risk of chronic spinal drug delivery. In the presence of possible infection, chronic spinal drug delivery population data on CSF chemistry are important for diagnosis. Bacterial infections result in increased protein, decreased glucose and increased WBC.22 Our study suggests that chronic spinal drug delivery does not alter protein, glucose or WBC and an abnormality in any of these markers suggests pathology. Further diagnostic evaluation is warranted.
Theoretical Consideration of Spinal Drug Disposition
A question we sought to address was the magnitude of the distribution of the MOR from the catheter tip. As the patient cohort received a variety of doses and concentrations, we had to rely on a normalization of the expected concentrations using the assumption that the concentrations would vary linearly with the infusion dose. This assumption is supported by the data in Figure 1. A second variable was the role of infusion rate. As noted, there was no evident covariance of measured concentration of the normalized concentration with rate. Though unexpected, this lack of effect suggests that over the range of 0.1 to 1 mL/day there is no significant effect upon distribution, e.g., higher rates would likely be required to force a greater redistribution with a given infusion. Ignoring rate, we found that there was a significant correlation between sample site and local concentration. The decline over several segments from the infusion site suggests that even at equilibrium there is a prominent localization of the infusate to the catheter tip. Intuitively, the steepness of this gradient can be appreciated by noting that at the catheter tip, the concentration of drug being delivered in a 20 mg/mL solution would approach a theoretical 20,000 μg/mL. Yet by 5 segments away the estimated concentration has already declined to a normalized value of 2.5 μg/mL. Such steep rostrocaudal gradients with low rate chronic infusions have been systematically described in the elegant porcine work of Flack and Bernards.28,29
An interesting caveat to this analysis is the rationale behind the use of a normalizing protocol (dividing each sample concentration by the respective daily infusion dose). Consideration of this strategy emphasizes that it is the same formula used to calculate clearance. The calculations to normalize CSF drug concentrations divide concentration (ng/ml) by infusion rate (mg/min). The result is actually a measure of clearance (ml/min). How can clearance vary as a function of distance from the tip of the intrathecal catheter? During a typical IV infusion, drug concentrations achieve equilibrium throughout the vascular space, so clearance is the same no matter where one samples. We would note, however, that in the clearance model, we are assuming all of the drug flows through all of the system (e.g., the blood after IV delivery); therefore, the “area under the curve” for drug concentration over time will be approximately the same no matter where you sample. However, we believe that the lumbar intrathecal space is largely a model of diffusion from a point source (the catheter tip) in a poorly mixed system. This was the conclusion reached by Shafer et al.17 in studying human intrathecal neostigmine kinetics. They specifically note that this decline in concentration at the progressively distant site reflects the role played by local dilution as the drug diffuses as well as the local diffusion (clearance) into tissue/meninges. In such a system, the concentration gradient is due to a series of segmental equilibria where a local proximal drug concentration in CSF is the driving force for diffusion into a distal segment of CSF. Serial dilution plays a major role in defining the changes in local CSF drug concentration, and the concentration of the infused drug will decrease as the inverse of the cube of the distance. This dilution is a first order process, (e.g., meaning that the rate of diffusion and hence concentration at a distant site will be directly proportional to concentration). Hence it is not unreasonable in a simple model where passive forces and local dilution are considered to play an important role that one may compare those systems by dividing by the dose that each receives, e.g., a normalization. Thus, dividing the samples of CSF concentration of MOR by the daily dose shows a significantly negative slope for MOR in CSF, but a zero slope for the plasma concentrations (though there is a clear relationship between the intrathecal dose and the measured plasma concentrations). This reflects the comments made above that the assessment of clearance assumes the homogenous distribution of the drug over time (where sampling anywhere in the arterial tree will give the same value). Two specific points also support the role of a local decline in MOR concentrations leading to the observed gradient, as compared to a change in MOR clearance from the CSF space. First, the sampling site was always the same (e.g L5/S1). It was the infusion site that varied across patients. Hence whatever accounts for the difference it cannot be ascribed to differences in MOR clearance at different sampling sites. Second, we may consider and compare the segmental distribution of the conjugated metabolites. Here we observed in contrast to MOR, that no matter where sampled, the observed concentrations of M3G or M6G were the same (when normalized by MOR infusion dose). This difference between the distribution of MOR and its metabolites in the CSF further support the comment that MOR delivered at a site shows a significant decline in concentration at increasing distances from the catheter tip.
Changes in Drug Delivery and CSF/Plasma Concentrations
Catheter failure
An important premise is that intrathecally delivered MOR will distribute into the CSF and then into plasma. Given that neuraxial metabolism plays a minor role in drug clearance from the CSF, it is not surprising that plasma concentrations co-vary with dose. However, with failure of intrathecal delivery, it would be predicted that the CSF concentrations will be unexpectedly low and the plasma concentrations normal. In the course of this study, patients #4 and #7 were observed to have unexpectedly low CSF MOR concentrations relative to the concentrations projected on the basis of their daily infusion dose (Table 3). As noted in the results, both of these subjects were determined to have malfunctioning catheters.
Granuloma
The present series of 18 patients revealed a single patient with a granuloma as confirmed by MRI. The incidence of such masses range from 3 in 7 in one series 30 to a population incidence of 0.1%.31 Preclinical studies have emphasized that at a dose of 12–18 mg/ml/day or higher, there is a 100 % incidence of granulomas in the dog 12 and sheep.32 Systematic studies suggested that the risk of granuloma formation is correlated with local CSF concentrations.33 Interestingly, sampling lumbar CSF in dogs proximal to the catheter tip have shown in acute pharmacokinetic studies that at the 12 mg/ml/day dose, CSF concentrations were on the order of 42,000 ng/mL.13 Interestingly, we emphasize that in the present case, the patient displaying the granuloma received a daily dose of 36 mg/day. The CSF concentration calculated from the regression analysis is approximately 40 mcg/ml at the sampling site, an amount that caused granulomas in dogs. Interestingly, the original studies found that dogs developing granulomas had strikingly low MOR concentrations in CSF but normal concentrations in plasma. Perhaps intrathecal MOR is cleared rapidly in the vicinity of a granuloma, because there is increased vascularity or compromise of the dura. Patient #6 was noted to have a much lower than predicted CSF MOR concentration (Figure 4). This discrepancy prompted the diagnostic MRI. Interestingly, this patient’s estimated MOR concentrations should have made her CSF concentrations the highest of the group examined. These findings have two implications. First, it suggests that the important variable toxicity arises not directly from the total dose or the infusion rate, but in fact the local concentration of drug to which the adjacent tissue is exposed (and the time for which that exposure occurs). Second, we accordingly suggest that the algorithm for converting preclinical safety data between species is the local concentration achieved by any given delivery protocol.
Intrathecal Morphine Metabolites
MOR is metabolized in the liver and central nervous system to M3G, and M6G by uridine-5′-diphosphate (UDP) glucuronosyltransferase.20 M6G, but not M3G, possesses potent opioid activity.34 Earlier work has indicated that the 3 conjugated metabolites of many opiates, including MOR has at the spinal level a potent pro-allodynic, strychnine-like effect.35 The mechanism of this action is not completely clear, but interesting observations have implicated interactions with spinal glia.36 In the present work, significant concentrations of both M3G and M6G were observed, with the later exceeding those concentrations seen with the former. These results and their concentrations were proportional to the measured concentrations of CSF MOR and co-varied with the intrathecal dose. The proportions observed in these human studies are strikingly similar to those ratios reported in canine chronic intrathecal MOR infusion sampling studies.13 There is significant controversy as to the degree to which CSF metabolite concentrations after neuraxial MOR delivery reflect a peripheral metabolism of redistributed MOR or a neuraxial conversion. Although MOR glucuronidation has been demonstrated in human brain tissue,19 the synthetic capacity is very low compared to that of the liver. There is little doubt that the conjugated metabolites are strongly excluded by the CNS. Nevertheless normalizing for the MOR concentration, the metabolite concentrations are considerably higher in the plasma than in the CSF. This supports the possibility that while the brain may indeed be capable of generating these metabolites, the possibility of a peripheral enzymatic conversion and redistribution to the CSF cannot be excluded. Furthermore, as discussed above, we observed that no matter where CSF was sampled, the observed concentrations of M3G or M6G were the same (when normalized by infusion dose). These observations are consistent with the hypothesis that the primary source of these conjugates is peripheral to the spinal cord and that it is the diffusion of the M3G/M6G all along the neuraxis that results in the low but evenly distributed metabolites. The absolute concentrations of M3G/M6G observed in these studies were less by an order of magnitude from those which have been previously reported in patients receiving chronic infusion.18 Interestingly, in those studies, unlike in the present work they found no covariance between metabolites and dosing.
In conclusion, this study demonstrated that the chronic infusion of MOR results in high concentrations of MOR which correlate with infusion dose and the presence of the conjugated metabolites. The chronic delivery of intrathecal MOR has no prominent effects on CSF chemistry. In spite of the continued infusion, these studies support a significant rostrocaudal gradient as one samples at increasing distances from the catheter tip. The study also presents an interesting case of the changes in CSF MOR concentrations that occur in the presence of a granuloma. The extremely low CSF MOR concentrations in correlation with MOR dose in the presence of a granuloma are consistent with the preclinical studies. It should be stressed that the above analysis, though revealing significant changes, is based on the results from only 12 successfully sampled patients with appropriately placed catheters. Negative findings have to be interpreted carefully considering the small sample size (false negative due to lack of power, e.g., patient characteristics and daily MOR dose or CSF MOR concentrations. Additional patients would be beneficial to confirm the observations.
Acknowledgments
Funding: Medtronics corporation (MSW) and the National Institutes of Health: NIH-DA15353 Bethesda, MD (TLY).
We would like to thank Medtronic, Inc. for their assistance in performing the assays.
Footnotes
Reprints will not be available from the authors.
Portions of these data was presented as a poster at the 2003 American Pain Society Annual Meeting
DISCLOSURES:
Name: Mark Wallace, MD
Contribution: This author helped prepare the manuscript.
Attestation: Dr. Mark Wallace approved the final manuscript, reviewed the data and analysis and maintained the study records.
Conflicts of Interest: Research Support from Medtronics, Inc.
Name: Tony L. Yaksh, PhD
Contribution: This author helped prepare the manuscript.
Attestation: Dr. Tony Yaksh approved the final manuscript, reviewed the data and analysis.
Conflicts of Interest: This author has no conflicts of interest to declare.
Contributor Information
Mark Wallace, Department of Anesthesiology, University of California San Diego California, La Jolla, California.
Tony L. Yaksh, Department of Anesthesiology, University of California San Diego California, La Jolla, California.
References
- 1.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–8. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- 2.Onofrio BM, Yaksh TL, Arnold PG. Continuous low-dose intrathecal morphine administration in the treatment of chronic pain of malignant origin. Mayo Clin Proc. 1981;56:516–20. [PubMed] [Google Scholar]
- 3.Coombs D, Maurer L, Suanders R, Gaylor M. Outcomes and complications of continuous intraspinal narcotic analgesics for cancer pain control. J Clin Oncol. 1984;2:1414–20. doi: 10.1200/JCO.1984.2.12.1414. [DOI] [PubMed] [Google Scholar]
- 4.Krames ES, Gershow J, Glassberg A, Keneflick T, Lyons A, Taylor P, Wilkie D. Continuous infusion of spinally administered narcotics for the relief of pain due to malignant disorders. Cancer. 1985;56:696–702. doi: 10.1002/1097-0142(19850801)56:3<696::aid-cncr2820560343>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Onofrio BM, Yaksh TL. Long-term pain relief produced by intrathecal morphine infusion in 53 patients. J Neurosurg. 1990;72:200–9. doi: 10.3171/jns.1990.72.2.0200. [DOI] [PubMed] [Google Scholar]
- 6.Follett KA, Hitchon PW, Piper J, Kumar VGC, Jones MP. Response of intractable pain to continuous intrathecal morphine: a restrospective study. Pain. 1992;49:21–5. doi: 10.1016/0304-3959(92)90183-C. [DOI] [PubMed] [Google Scholar]
- 7.Krames ES, Lanning RM. Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. J Pain Sympt Manage. 1993;8:539–48. doi: 10.1016/0885-3924(93)90083-8. [DOI] [PubMed] [Google Scholar]
- 8.Wallace MS, Yaksh TL. Long-term intraspinal drug therapy: A review. Reg Anesth and Pain Med. 2000;25:117–57. doi: 10.1053/rapm.2000.0250117. [DOI] [PubMed] [Google Scholar]
- 9.Moore R, Bullingham R, McQuay H, Allen M, Baldwin D, Cole A. Spinal fluid kinetics of morphine and heroin. Clin Pharmacol Ther. 1984;35 doi: 10.1038/clpt.1984.6. [DOI] [PubMed] [Google Scholar]
- 10.Ionescu T, Drost R, Roelofs J, Winckers E, Taverne R, van Maris A, van Rossum J. The pharmacokinetics of intradural morphine in major abdominal surgery. Clinical Pharmacokinetics. 1988;14:178–86. doi: 10.2165/00003088-198814030-00006. [DOI] [PubMed] [Google Scholar]
- 11.Bigler D, Christensen C, Eriksen J, Jensen N. Morphine, morphine-6-glucuronide and morphine-3-glucuronide concentrations in plasma and cerebrospinal fluid during long-term high-dose intrathecal morphine administration. Pain. 1990;41:15–8. doi: 10.1016/0304-3959(90)91103-P. [DOI] [PubMed] [Google Scholar]
- 12.Yaksh TL, Horais KA, Tozier NA, Allen JW, Rathbun M, Rossi SS, Sommer C, Meschter C, Richter PJ, Hildebrand KR. Chronically infused intrathecal morphine in dogs. Anesthesiology. 2003;99:174–187. doi: 10.1097/00000542-200307000-00028. [DOI] [PubMed] [Google Scholar]
- 13.Allen J, Horais K, Tozier N, Wegner K, Corbeil J, Mattrey R, Rossi S, Yaksh T. Time course and role of morphine dose and concentration in intrathecal granuloma formation in dogs: a combined magnetic resonance imaging and histopathology investigation. Anesthesiology. 2006;105:581–9. doi: 10.1097/00000542-200609000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Flack S, Anderson C, Bernards C. Morphine distribution in the spinal cord after chronic infusion in pigs. Anesth Analg. 2011;112:460–4. doi: 10.1213/ANE.0b013e318203b7c0. [DOI] [PubMed] [Google Scholar]
- 15.Bernards C. Cerebrospinal fluid and spinal cord distribution of baclofen and bupivacaine during slow intrathecal infusion in pigs. Anesthesiology. 2006;105:169–78. doi: 10.1097/00000542-200607000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Ummenhofer W, Arends R, Shen D, Bernards C. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000;92:739–53. doi: 10.1097/00000542-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Shafer S, Risenach J, Hood D, Tong C. Cerebrospinal fluid pharmacokinetics and pharmacodynamics of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1998;89:1074–788. doi: 10.1097/00000542-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Dennis G, Soni D, Dehkordi O, Millis R, James H, West W, Taylor R. Analgesic responses to intrathecal morphine in relation to CSF concentrations of morphine-3, beta-glucuronide and morphine-6, beta-glucuronide. Life Sci. 1999;64:1725–31. doi: 10.1016/s0024-3205(99)00110-1. [DOI] [PubMed] [Google Scholar]
- 19.Wahlstr6m A, Winblad B, Bixo M, Rane A. Human brain metabolism of morphine and naloxone. Pain. 1988;35:121–7. doi: 10.1016/0304-3959(88)90219-9. [DOI] [PubMed] [Google Scholar]
- 20.Christrup L. Morphine metabolites. Acta Anaesthesiol Scand. 1997;41:116–22. doi: 10.1111/j.1399-6576.1997.tb04625.x. [DOI] [PubMed] [Google Scholar]
- 21.Naidong W, Lee J, Jiang X, Wehling M, Hulse J, Lin P. Simultaneous assay of morphine, morphine-3-glucuronide and morphine-6-glucuronide in human plasma using normal-phase liquid chromatography-tandem mass spectrometry with a silica column and an aqueous organic mobile phase. J Chromatogr B Biomed Sci Appl. 1999;735:255–69. doi: 10.1016/s0378-4347(99)00429-6. [DOI] [PubMed] [Google Scholar]
- 22.Beers MH, Berkow R. The Merck Manual of Diagnosis and Therapy. Whitehous Station, New Jersey: Merck Research Laboratories; 1999. [Google Scholar]
- 23.Chiari A, Yaksh TL, Myers RR, Provencher J, Moore L, Lee CS, Eisenach JC. Preclinical toxicity screening of intrathecal adenosine in rats and dogs. Anesthesiology. 1999;91:824–32. doi: 10.1097/00000542-199909000-00035. [DOI] [PubMed] [Google Scholar]
- 24.Sabbe MB, Grafe MR, Mjanger E, Tiseo PJ, Hill HF, Yaksh TL. Spinal delivery of sufentanil, alfentanil, and morphine in dogs. Physiologic and toxicologic investigations. Anesthesiology. 1994;81:899–920. doi: 10.1097/00000542-199410000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Yaksh TL, Grafe MR, Malkmus S, Rathbun ML, Eisenach JC. Studies on the safety of chronically administered intrathecal neostigmine methylsulfate in rats and dogs. Anesthesiology. 1995;82:412–27. doi: 10.1097/00000542-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Yaksh TL, Rathbun M, Jage J, Mirzai T, Grafe M, Hiles RA. Pharmacology and toxicology of chronically infused epidural clonidine. HCl in dogs. Fundam Appl Toxicol. 1994;23:319–35. doi: 10.1006/faat.1994.1112. [DOI] [PubMed] [Google Scholar]
- 27.Sabbe MB, Grafe MR, Pfeifer BL, Mirzai TH, Yaksh TL. Toxicology of baclofen continuously infused into the spinal intrathecal space of the dog. Neurotoxicology. 1993;14:397–410. [PubMed] [Google Scholar]
- 28.Flack S, Bernards C. Cerebrospinal fluid and spinal cord distribution of hyperbaric bupivacaine and baclofen during slow intrathecal infustion in pigs. Anesthesiology. 2010;112:165–73. doi: 10.1097/ALN.0b013e3181c38da5. [DOI] [PubMed] [Google Scholar]
- 29.Flack S, Bernards C. Morphine distribution following chronic intrathecal infusion in ambulatory pigs. Anesthesiology. 2010;A198:2010b. [Google Scholar]
- 30.McMillan M, Doud T, Nugent W. Catheter-associated masses in patients receiving intrathecal analgesic therapy. Anesth Analg. 2003;96:186–90. doi: 10.1097/00000539-200301000-00039. [DOI] [PubMed] [Google Scholar]
- 31.Coffey RJ, Burchiel K. Inflammatory mass lesions associated with intrathecal drug infusion catheters: Report and observations on 41 patients. Neurosurgery. 2002;50:78–87. doi: 10.1097/00006123-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Gradert T, Baze W, Satterfield W, Hildebrand K, Johansen M, Hassenbusch S. Safety of chronic intrathecal morphine infusion in a sheep model. Anesthesiology. 2003;99:188–98. doi: 10.1097/00000542-200307000-00029. [DOI] [PubMed] [Google Scholar]
- 33.Allen J, Horais K, Tozier N, Yaksh T. Opiate pharmacology of intrathecal granulomas. Anesthesiology. 2006;105:590–8. doi: 10.1097/00000542-200609000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Wittwer E, Kern S. Role of morphine’s metabolities in analgesia: concepts and controversies. AAPS J. 2006;8:e348–52. doi: 10.1007/BF02854905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaksh T, Harty G. Pharmacology of the allodynia in rats evoked by high dose intrathecal morphine. J Pharmacol Exp Ther. 1988;244:501–7. [PubMed] [Google Scholar]
- 36.Hutchinson M, Shavit Y, Grace P, Rice K, Majer S, Watkins L. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]


