Abstract
Arterial hypertension is a major risk factor for ischemic stroke. However, the management of preexisting hypertension is still controversial in the treatment of acute stroke in hypertensive patients. The present study evaluates the influence of preserving hypertension during focal cerebral ischemia on stroke outcome in a rat model of chronic hypertension, the spontaneously hypertensive rats (SHR). Focal cerebral ischemia was induced by transient (1-hour) occlusion of the middle cerebral artery, during which mean arterial blood pressure was maintained at normotension (110-120 mmHg, group 1, n=6) or hypertension (160-170 mmHg, group 2, n=6) using phenylephrine. T2-, diffusion- and perfusion-weighted MRI were performed serially at five different time points: before and during ischemia, and at 1, 4 and 7 days after ischemia. Lesion volume and brain edema were estimated from apparent diffusion coefficient maps and T2-weighted images. Regional cerebral blood flow (rCBF) was measured within and outside the perfusion deficient lesion and in the corresponding regions of the contralesional hemisphere. Neurological deficits were evaluated after reperfusion. Infarct volume, edema, and neurological deficits were significantly reduced in group 2 versus group 1. In addition, higher values and rapid restoration of rCBF were observed in group 2, while rCBF in both hemispheres was significantly decreased in group 1. Maintaining preexisting hypertension alleviates ischemic brain injury in SHR by increasing collateral circulation to the ischemic region and allowing rapid restoration of rCBF. The data suggest that maintaining preexisting hypertension is a valuable approach to managing hypertensive patients suffering from acute ischemic stroke.
Keywords: arterial spin labeling, cerebral blood flow, hypertension, ischemic stroke, magnetic resonance imaging, spontaneously hypertensive rats
1. Introduction
Arterial hypertension is the major risk factor of ischemic stroke and is associated with poor prognosis, including early and late death, neurological impairments, cerebral edema and hemorrhage, and recurrence (Leonardi-Bee et al., 2002). Even though progressive lowering of blood pressure reduces the incidence of stroke through long-term administration of antihypertensive agents, the management of preexisting hypertension is still controversial in patients with acute ischemic stroke because of the lack of unambiguous data (Adams et al., 2007).
During ischemic stroke, the presence of severe hypertension may worsen secondary brain injury by increasing cerebral edema or hemorrhage (Ayata, 2002; Cole et al., 1990b), and systolic/diastolic blood pressure > 185/110 mm Hg is a contraindication to recombinant tissue plasminogen activator administration (Brott et al., 1998). On the other hand, rapid lowering of blood pressure is discouraged because it may worsen outcome by reducing perfusion pressure in the salvageable penumbra area. A metaregression analysis of the relationship between changes in blood pressure and functional outcome showed that large drops or increases in blood pressure are associated with a higher risk of poor outcome (Geeganage and Bath, 2009). In that study, modest reductions in blood pressure (14 to 15 mm Hg) were associated with the lowest risk of death or dependency. Due to the lack of consensus data, the current guidelines recommend that antihypertensive treatments should be withheld unless the blood pressure is > 220/120 mm Hg (systolic/diastolic) (Adams et al., 2007).
Because autoregulation of cerebral blood flow (CBF) is shifted to the right in patients with chronic hypertension, they are more vulnerable to emergency antihypertensive therapy (Strandgaard et al., 1973). In addition, the change of CBF in acute ischemic stroke depends on arterial blood pressure because of the impairment of cerebral autoregulation following stroke (Symon et al., 1976). Therefore, we hypothesized that maintaining hypertension during ischemic stroke has positive effects in hypertensive rats.
Previous studies have shown that hypertension, which was induced by drugs during occlusion or reperfusion of cerebral artery, is beneficial for reducing ischemic area in normotensive animals (Chileuitt et al., 1996; Cole et al., 1992; Drummond et al., 1989; Ishikawa et al., 2009; Shin et al., 2008; Smrcka et al., 1998). However the effect of maintaining preexisting hypertension during ischemic stroke has not been systemically evaluated in hypertensive animals and humans. To verify the influence of preserving hypertension on clinical outcome of chronically hypertensive patients with acute ischemic stroke, we monitored changes of infarct volume, brain edema, neurological deficits, and CBF in spontaneously hypertensive rats (SHR), an important experimental model in the study of stroke and other cerebrovascular diseases (Amenta et al., 2003), during and following transient middle cerebral artery occlusion (MCAO).
2. Results
2.1. Physiological parameters
There were no mortalities associated with the present study. In all rats, physiological parameters were maintained within normal range before, during, and after MCAO (Table 1). There were no significant differences in the parameters between two groups except for the mean arterial blood pressure (MABP) measured during MCAO. MABP of the rats in group 2 was significantly higher than that of group 1 during MCAO (P < 0.01). However, MABP was not different between groups after discontinuing injection of phenylephrine (P > 0.05).
Table 1.
Physiological parameters measured before, during, and after middle cerebral artery occlusion (MCAO)
| MABP (mm Hg) |
pH | PaO2 (mm Hg) |
PaCO2 (mm Hg) |
Temperature (°C) |
|
|---|---|---|---|---|---|
| Before MCAO | |||||
| Group 1 | 122 ± 14 | 7.43 ± 0.04 | 165 ± 7 | 27 ± 3 | 38.2 ± 0.5 |
| Group 2 | 111 ± 10 | 7.39 ± 0.04 | 155 ± 9 | 29 ± 2 | 37.8 ± 0.2 |
| During MCAO | |||||
| Group 1 | 119 ± 21 | 7.39 ± 0.03 | 165 ± 20 | 31 ± 3 | 38.2 ± 0.3 |
| Group 2 | 158 ± 8** | 7.36 ± 0.04 | 160 ± 10 | 34 ± 4 | 38.2 ± 0.4 |
| Reperfusion | |||||
| Group 1 | 111 ± 22 | 7.36 ± 0.05 | 169 ± 17 | 35 ± 4 | 38.4 ± 0.5 |
| Group 2 | 103 ± 21 | 7.35 ± 0.02 | 166 ± 10 | 35 ± 5 | 38.0 ± 0.5 |
MABP: mean arterial blood pressure;
P < 0.01 for differences between two groups (two-way repeated measures ANOVA with Bonferroni post-hoc test)
2.2. Evolution of ischemic lesion volume and brain edema
Lesion volumes were significantly smaller in group 2 than in group 1 at each measurement time (during MCAO and Day 1: P < 0.05, Day 4 and 7: P < 0.01; Fig. 1). The difference of mean lesion volumes between two groups increased with time (during MCAO: 3.11 %; Day 1: 6.12 %; Day 4: 7.8 %; Day 7: 9.05 %). Maximal lesion volumes were observed at Day 4 (group 1: 33.96 %, group 2: 26.16 %), but the evolution of lesion volumes was not significant in either group (during MCAO vs. Day 4: P > 0.05).
Fig. 1.
Temporal evolution of lesion volumes estimated after transient ischemia in SHR in group 1 (normotension during MCAO) and group 2 (hypertension during MCAO). Lesion volumes were significantly smaller in group 2 compared to group 1, indicating a benefic effect of preserving hypertension for 7 days after ischemia. * = P < 0.05; ** = P < 0.01 between groups 1 and 2 (two-way repeated measures ANOVA with Bonferroni post-hoc test).
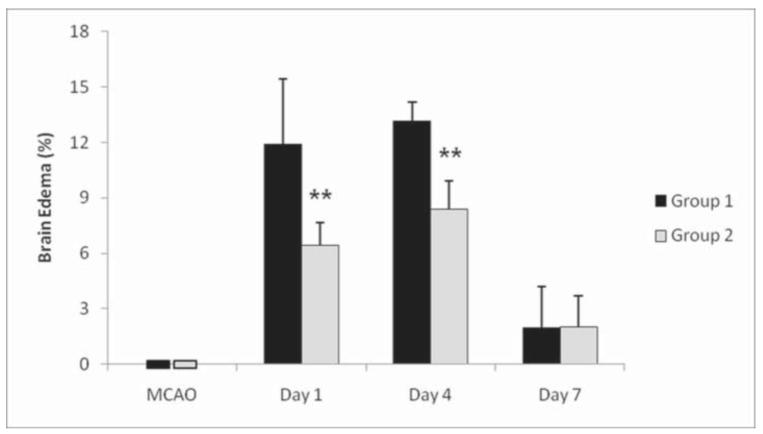
While acute brain edema was not observed in either group during MCAO, brain edema was present after at Day 1 and was largest at Day 4 (group 1: 13.13 ± 1.04 %; group 2: 8.37 ± 1.52 %). Group 2 had a significant smaller edema compared to group 1 at Days 1 and 4 (P < 0.01; Fig. 2). At Day 7, edema was greatly decreased in both groups and not different from the extent measured during MCAO (P>0.05).
Fig 2.
Temporal evolution of brain edema measured following transient ischemia in SHR. Maintenance of hypertension during MCAO (group 2) induced significantly smaller brain edema compared allowing normotension during brain ischemia (group 1) at 1 day and 4 days after MCAO. In both groups, edema resolved by 7 days after ischemia. ** = P < 0.01 (two-way repeated measures ANOVA with Bonferroni post-hoc test).
Fig. 3 shows representative ADC maps, T2-weighted images and CBF maps demonstrating the temporal evolution of ischemic lesions, along with the regional perfusion status of brain tissue. In both groups, ADC deficient and hyperintense lesions were observed in cortical and subcortical areas. However, group 1 had larger lesions compared to group 2, especially in the dorsolateral cortex and in the basal ganglia. In addition, severely compressed ventricles and mid-line shift due to edema were more prominent in group 1.
Fig 3.
Representative ADC maps, T2-weighted images, and CBF maps demonstrating the temporal evolution of ischemic lesions and rCBF obtained in SHR without phenylephrine injection (group 1) and with phenylephrine-induced maintenance of hypertension (group 2) during MCAO. Maintaining hypertension induced smaller regions of ADC deficit, as well as smaller hyperintense lesions and less edema in group 2 compared to group 1 during MCAO and at 1 day, 4 days and 7 days after MCAO. In addition, while rCBF in both hemispheres was significantly decreased in group 1, higher values and rapid restoration of rCBF were observed in group 2. The color bar expresses the CBF values in mL/100 g/min.
2.3. Evolution of neurological deficits
In both groups, neurological deficits were observed after reperfusion. Neurological score was the highest at Day 1 (group 1: 6.7 ± 0.8, group 2: 4.0 ± 1.6) and significantly lower in group 2 than that in group 1 at each measurement time (Day 1 and 4: P < 0.05, Day 7: P < 0.01; Fig. 4). Even though neurological deficits gradually improved with time, they did not disappear in either group by Day 7, the last day of observation in the present study (Day 1 vs. Day 7: P > 0.05).
Fig 4.
Temporal evolution of neurological scores evaluated following transient ischemia in SHR. Maintaining hypertension during MCAO led to lower neurological deficits in group 2 compared to group 1, indicating a benefic effect of preserving hypertension in the acute phase of stroke. * = P < 0.05; ** = P < 0.01 (Mann-Whitney U-test).
2.4. Evolution of rCBF
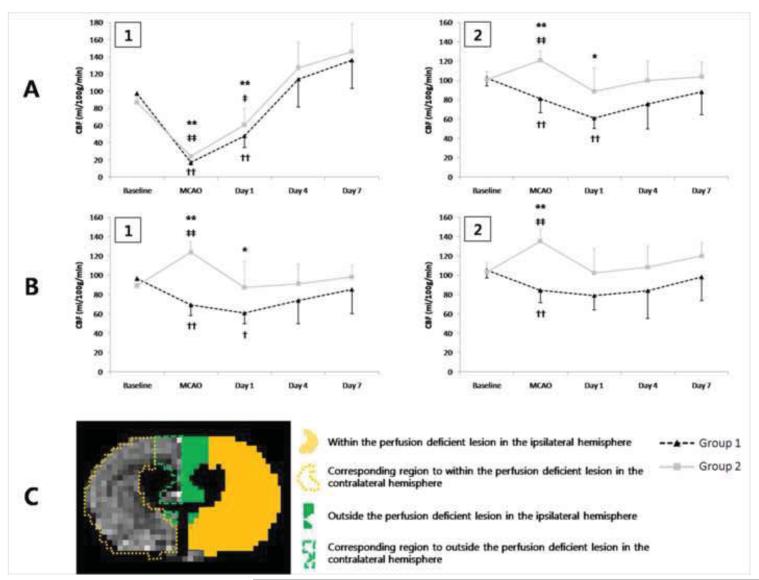
Representative CBF maps demonstrating the temporal evolution of rCBF are shown in Fig. 3. Fig. 5 shows the temporal evolution of rCBF measured within and outside the perfusion deficient lesion of the ipsilateral hemisphere, and in corresponding regions of the contralateral hemisphere. Even though perfusion-deficient lesion volumes measured during MCAO were not different between the two groups (group 1: 32.78 ± 3.72 %, group 2: 33.85 ± 2.19 %; P > 0.05), diffusion-perfusion mismatch area was significantly larger in group 2 (group 1: 6.65 ± 5.36 %, group 2: 10.87 ± 1.28 %; P < 0.05). Within the perfusion deficient lesion (Fig. 5A-1), rCBF measured before ischemia was not different between the two groups (P > 0.05). MCAO significantly reduced rCBF to 17.7 % (group 1) and 27.9 % (group 2) of pre-ischemic values (P < 0.01). Decreased rCBF within the perfusion deficient lesion was not restored until Day 4 (baseline vs. Day 1; group 1: P < 0.01, group 2: P < 0.05) (Fig. 5A-1). rCBF in group 2 was significantly higher than in group 1 only during MCAO and at Day 1 (Fig. 5A-1, P < 0.01), but not different at Day 4 and Day 7 (P > 0.05).
Fig 5.
Temporal evolution of rCBF measured within (1) and outside (2) the perfusion deficient lesion of the ipsilateral hemisphere (A), as well as in the corresponding regions of the contralateral hemisphere (B). (C) A representative image of the CBF map shows 4 ROIs outlining those areas. * = P < 0.05 and ** = P < 0.01 for differences between groups; † P < 0.05 and †† P < 0.01 for comparisons to baseline rCBF within group 1; ‡ P < 0.05 and ‡‡ P < 0.01 for comparisons to baseline rCBF within group 2 (two-way repeated measures ANOVA with Bonferroni post-hoc test).
Outside the perfusion deficient lesion (Fig. 5A-2), rCBF of group 2 was significantly increased during injection of phenylephrine (baseline vs. during MCAO: P < 0.01), but returned to pre-ischemic values after reperfusion (baseline vs. Day 1, 4, and 7: P > 0.05, Fig 5A-2). On the other hand, in group 1 rCBF outside the perfusion deficient lesion was significantly decreased during MCAO (baseline vs. during MCAO: P < 0.01) and it did not return to pre-ischemic values until Day 4 (baseline vs. Day 1: P < 0.01). When compared across the two experimental groups, rCBF outside the lesion volume was significantly higher in group 2 than in group 1 during MCAO (P < 0.01) and at Day 1 (P < 0.05), but this difference disappeared at Day 4 and Day 7 (P > 0.05). rCBF was also measured in the contralateral hemisphere in analogous regions corresponding to within (Fig. 5B-1) and outside the perfusion deficient lesion (Fig. 5B-2). Contralateral rCBF was significantly increased in group 2 during injection of phenylephrine (baseline vs. during MCAO: P < 0.01), but returned to pre-ischemic values after reperfusion (baseline vs. Day 1, 4, and 7: P > 0.05). Interestingly, rCBF measured both in the contralateral and ipsilateral hemispheres was significantly decreased in group 1 during MCAO (baseline vs. during MCAO: P < 0.01). Contralateral rCBF in the region corresponding to within the perfusion deficient lesion remained decreased in group 1 until Day 1 (baseline vs. Day 1: P < 0.05). When compared across the two experimental groups, contralateral rCBF was significantly higher in group 2 than in group 1, during MCAO (P < 0.01) and at Day 1 (P < 0.05). This difference disappeared after Day 1 in the region corresponding to outside the perfusion deficient lesion (P > 0.05), or Day 4 in the region corresponding to within the perfusion deficient lesion (P > 0.05).
3. Discussion
The present study shows that maintaining pre-existing hypertension during arterial occlusion results in reduced ischemic lesion volume, brain edema, and neurological deficits in SHR, a well-established animal model of chronic hypertension. In addition, phenylephrine-induced hypertension caused increased rCBF outside the infarct area, and rapid restoration of decreased rCBF within the infarct area in treated animals. Taken together, these findings suggest that sustaining elevated blood pressure is a benefic approach in the management of acute stroke in patients with chronic hypertension. In addition, the information gained in the present study may be helpful for managing hypertensive patients with acute ischemic stroke before administration of thrombolytic agents, as hypertension was induced only during the period of ischemia.
In the present study, all experiments were carried out under isoflurane anesthesia. Even though isoflurane has a strong effect on rCBF and vascular reactivity (Leoni et al., 2011), the use of isoflurane in the present study was necessary to allow monitoring of the temporal changes in lesion volume, edema, and rCBF by MRI. Because isoflurane decreases systemic blood pressure to normotensive levels in SHR by systemic vasodilation, phenylephrine was used during unilateral MCAO to counteract the vasodilatory effects of isoflurane and sustain elevated blood pressure. Phenylephrine is a selective α1-adrenergic agonist that increases arterial blood pressure by peripheral vasoconstriction. Even though there is a concern that phenylephrine may induce cerebral vasoconstriction, previous reports have shown that the brain has relatively few α1-adrenergic receptors and that phenylephrine does not influence CBF in the conscious rat (Bevan et al., 1987; Sokrab et al., 1989). In addition, a previous study showed that isoflurane-induced vasodilation is not mediated by inhibition of α1-adrenergic responsiveness (Schwinn et al., 1990), therefore isoflurane may not suppress the action of phenylephrine. In fact, phenylephrine has been safely used to increase blood pressure both in experimental studies of stroke and in clinical patients (Chalela et al., 2005; Cole et al., 1992, 1990b; Ishikawa et al., 2009; Rordorf et al., 2001; Shin et al., 2008). In the present study, rCBF in the healthy contralesional hemisphere did not decrease during administration of phenyephrine, suggesting that phenylephrine does not act as a cerebral vasoconstrictor in SHR, and that it does not impair autoregulation.
A positive effect of phenylephrine-induced hypertension in ischemic stroke has been previously reported (Cole et al., 1992; Drummond et al., 1989; Ishikawa et al., 2009; Shin et al., 2008; Smrcka et al., 1998). However, most of those studies induced hypertension after reperfusion and used normotensive animals. To the best of our knowledge, there have been only two reports evaluating the effect of maintaining hypertension during occlusion on the severity of ischemic stroke in hypertensive rats (Cole et al., 1990a; Harms et al., 2000). The present findings are in general agreement with the conclusions of both reports. Cole et al. (1990a) showed that hemodilution combined with phenylephrine-induced hypertension during 3 hours of MCAO reduced ischemic injury in SHR. That report, however, could not disambiguate the relative contribution of hemodilution versus maintenance of hypertension to the observed decrease in ischemic injury. The other study demonstrated that reduction of blood pressure to normotension using dihydralazine worsened neurological outcome and increased lesion volume in spontaneously hypertensive stroke-prone rats (SHR-SP), compared to animals in which the blood pressure was either maintained or mildly reduced (Harms et al., 2000), suggesting that acute treatment of hypertension negatively affects neurological outcome and worsens infarct volume in chronically hypertensive patients.
Induced hypertension has been postulated to be effective for diminishing ischemic injury by increasing collateral circulation and favorably redistributing rCBF in the infarct area (Cole et al., 1990a; Ishikawa et al., 2009; Shin et al., 2009; Smrcka et al., 1998). Several reports demonstrated increasing rCBF in the ischemic region using laser-Doppler flowmetry or diffusible tracers (Drummond et al, 1989; Ishikawa et al., 2009; Smrcka et al., 1998; Harms et al., 2000). However those techniques have some disadvantages, such as difficulties of periodic measurements during long-term period and calculating absolute value of CBF, therefore we used ASL technique to monitor the change of rCBF in the brain.
Usually cerebral ischemia disturbs autoregulation of CBF in the ischemic region because of maximal arteriolar vasodilatation and loss of the capacity for vasoconstriction (Waltz, 1968). Therefore arterial blood pressure may have a direct effect on CBF in acute ischemia. In this study, induced hypertension of group 2 significantly increased rCBF outside the perfusion deficient lesion during MCAO, which includes the area receiving collateral circulation from the contralateral cerebral arteries. Therefore, by maintaining hypertension, high collateral flow was induced in the border zone of the infarct, and this may contribute to relatively larger diffusion-perfusion mismatch area and higher rCBF within the perfusion deficient lesion of group 2 compared to group 1. Interestingly, rCBF of group 1 was significantly decreased in the contralateral side as well as the ipsilateral hemisphere. This finding shows that cerebral autoregulation could be lost in the non-ischemic area of SHR if blood pressure is decreased to normotensive levels during occlusion. This loss of autoregulation in the contralateral side may worsen decrease of collateral circulation to the ischemic hemisphere of group 1. One concern of maintaining hypertension is the possibility of aggravating cerebral edema by high blood pressure because autoregulation is lost in the ischemic region. In this study, lesion volumes on the DWI and T2-weighted images were significantly larger in group 1 than group 2. In addition, cerebral edema of group 1 was more prominent than that of group 2. These findings show that maintaining hypertension during occlusion reduces cytotoxic and vasogenic edema in SHR. Therefore relatively small injury of brain tissue and formation of brain edema may contribute to better neurologic functions in group 2 compared to group 1.
The present study has some limitations. First, the duration of maintaining hypertension and occlusion was relatively short. The infusion of thrombolytic agents within 3 hours of the onset symptoms is effective in restoring blood flow and improving stroke outcome in humans (Adams et al., 2007). In addition, a previous study has reported that the reduction of lesion volume is about half in the normotensive rabbits subjected to 2 hours of occlusion compared to the animals subjected to 1 hour occlusion (Smrcka et al., 1998). The effects of longer periods (up to 3 hours) of maintaining hypertension should be evaluated in a future study. Second, we relied on the vasodilatory effects of isoflurane rather than using a hypotensor to decrease blood pressure to normotensive levels in group 1. To mimic the clinical circumstances, the application of a hypotensor combined with an anesthetic drug that has little influence to blood pressure should be considered. In conclusion, the present data show that maintaining hypertension during 1 hour of occlusion alleviates ischemic brain injury in SHR by increasing collateral circulation to the ischemic region and rapid restoring of CBF after reperfusion. In addition, cerebral ischemia interfered with autoregulation of CBF in both hemispheres, when blood pressure was decreased to normotensive levels. Therefore, maintaining preexisting hypertension may contribute to better outcome of hypertensive patients suffering from acute ischemic stroke, and these results will be helpful for the management of clinical patients.
4. Experimental procedures
4.1. Animal Preparation
All procedures were performed in accordance with the institutional Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke, National Institutes of Health. Adult male SHR (n = 12, body weight 250 – 350 g, 13 – 18 weeks old) were purchased from Harlan laboratories, Somerville, NJ. All rats were housed under a 12 hours light/dark cycle and given free access to food and water. Each animal was anesthetized with 5% isoflurane in an induction chamber. General anesthesia was maintained by allowing the animals to freely breathe 2.5% isoflurane delivered in a mixture of 40% air, 40% nitrogen, and 20% oxygen during surgery using an open face mask. Body temperature was monitored and maintained at 37.5 °C using a thermostatically controlled heating pad (Gaymar Industries Inc., Orchard Park, NY). The tail artery was cannulated with PE-50 tube for continuous monitoring of arterial blood pressure (Biopac Systems Inc., Goleta, CA) and repeated sampling of arterial blood. Blood samples were collected before, during, and after MCAO to measure blood gases and pH (Radiometer America Inc., Westlake, OH). The lateral tail vein was cannulated with a 24-gauge intravenous catheter to inject normal saline or phenylephrine.
4.2. Surgical Procedures
Focal cerebral ischemia was induced on the right side by transient MCAO as previously described (Longa et al., 1989). Briefly, the right carotid bulb was exposed by a midline incision, and then the external carotid artery was transected and the internal carotid artery was carefully isolated. A 4-0 silicone-coated nylon suture (Doccol Corporation, Redlands, CA) was inserted into the internal carotid artery and advanced 19 – 20 mm until resistance was met. At 1 hour after MCAO, the suture was withdrawn to permit reperfusion. After evaluating blood pressure and sampling arterial blood gases, all catheters were removed, the suture site was closed, and rats were allowed to recover from anesthesia.
4.3. Experimental Protocol
SHR were assigned to one of two experimental groups: normotensive during stroke or hypertensive during ischemic stroke. In group 1 (normotension, n = 6), blood pressure was maintained at 110 – 120 mm Hg during MCAO under isoflurane inhalation. In group 2 (hypertension, n = 6), hypertension (160 – 170 mm Hg) was induced during ischemia by continuous injection of phenylephrine (15 – 20 g/kg/min) (Baxter Healthcare Corporation, Deerfield, IL). On the other hand, normal saline was infused into the normotensive group (injection rate: 10 ml/kg/hr) during occlusion. After reperfusion, injection of either phenylephrine or saline was discontinued.
4.4. Neurological Scoring
At 1 day, 4 days and 7 days after MCAO, neurological deficits were evaluated in both groups using a modified categorical rating scale, as described previously (Barone et al., 2001; Chu et al., 2008). Scoring was performed for postural reflexes (no deficit (0), contralateral forelimb flexion when suspended by the tail (1), reduced resistance to lateral push toward the contralateral side (1)), circling (absence (0), presence (2)), and proprioceptive placing of contralateral forelimb and hindlimb (complete immediate placing (0), incomplete and/or delayed placing (< 2 seconds) (1), absence of placing (2)). According to this scoring, a completely normal rat would earn a total score of 0, and a rat with the most severe deficits would earn a total score of 8. All scoring was performed by the same experienced observer, who was blinded to group assignment.
4.5. Magnetic Resonance Imaging
In both groups, magnetic resonance imaging (MRI) was serially performed at five different time points; before and during MCAO, then at 1 day, 4 days and 7 days after MCAO. Isoflurane anesthesia was maintained at 2% and vital signs were continuously monitored by end-tidal CO2, heart rate, and SPO2 using a capnograph and pulse oximeter (Surgivet, Waukesha, WI). Animals were placed on an MR-compatible cradle and their heads secured to a custom-designed head holder via ear pieces and a bite bar. MRI was acquired on a 7T/30 cm AVIII MRI system (Bruker Biospin Inc., Billerica, MA), equipped with a 15 cm gradient set of 450 mT/m strength (Resonance Research Inc., Billerica, MA). A home-built, transmit-only birdcage volume RF coil, 12 cm internal diameter, and a home-built, receive-only surface coil were used for all image acquisition. For arterial spin labeling (ASL), a home-built labeling coil was positioned under the neck of the animal approximately 2 cm away from the magnet’s isocenter, and connected to the second RF transmit channel of the spectrometer. All coils were equipped with active decoupling circuits to minimize coil-to-coil interferences during the labeling and imaging stages of the experiment, and to minimize off-resonance saturation of water in the acquisition region (Silva et al., 1995).
For each imaging sequence, 15 slices, with 1 mm each of thickness were acquired. Diffusion weighed imaging (DWI) was performed during MCAO using the following parameters: single-shot spin-echo echo-planar images (EPI), TR/TE = 6000/30 ms, matrix = 64 × 64, field of view (FOV) = 25.6 × 25.6 mm2, 1 direction, b-values = 0, 1600 s/mm2. From the DWI images, apparent diffusion coefficient (ADC) trace maps were calculated using computer software (DPTools, Denis Ducreux, Paris, France). T2-weighted images were acquired at 1 day, 4 days and 7 days after MCAO using a relaxation enhancement (RARE) sequence: TR/TE = 12000/74 ms, matrix = 128 × 128, FOV = 25.6 × 25.6 mm2. CBF measurements were made using a continuous arterial spin labeling (CASL) technique (Lu et al., 2010; Silva et al., 1995), with a spin-echo EPI sequence: TR/TE = 10000/30 ms, matrix = 64 × 64, FOV = 25.6 × 25.6 mm2. A post-labeling delay of 994 ms was employed to avoid intravascular contamination (Alsop and Detre, 1996). A labeling RF pulse of 8183 ms was applied in the presence of a longitudinal gradient Gz = 1 G/cm at the appropriate labeling frequency offset. Quantitative CBF maps were obtained by subtraction of the ASL images from control images using MATLAB (The MathWorks, Natick, MA). Following the imaging studies (7 days post-stroke), all rats were euthanized with potassium chloride (APP Pharmaceuticals, Schaumburg, IL) (100 mg/kg, IV).
4.6. Data Analysis
All acquired images were analyzed with MIPAV (Biomedical Imaging Research Services Section, National Institutes of Health, Bethesda, MD). Ischemic lesion during occlusion was determined on the ADC maps by using a 23% reduction in mean contralateral ADC (McCabe et al., 2009). After MCAO, lesion volumes were determined by manual tracing of the hyperintense lesions on the T2-weighted images. The corresponding ADC and T2 lesion volumes were then calculated by summing the abnormal area and multiplying by the slice thickness. Acquired lesion volumes were corrected for the space occupying effect due to edema and expressed as percentage of the hemispheric volume. Lesion volume with edema correction and brain edema were calculated as described previously (Gerriets et al., 2004). Perfusion deficient lesion was determined on all slices of the CBF map obtained during MCAO by using a 57% reduction relative to contralateral side (Meng et al., 2004). Diffusion-perfusion mismatch during occlusion was calculated as the difference between the perfusion deficit minus the ADC lesion area. The region of interest outlining (ROI) these areas were transferred to the corresponding CBF map obtained at different time points. Regional CBF (rCBF) values were measured within and outside the perfusion deficient lesion and in the corresponding region of the contralateral hemisphere.
4.7. Statistical analysis
Results were expressed as mean ± standard deviation. Non-parametric data, such as neurological scores, were evaluated by Mann-Whitney U-test and Wilcoxon signed rank test. An unpaired t-test was used for comparison of diffusion-perfusion mismatch between two groups. Serial changes of physiological parameters, lesion volumes, brain edema, and rCBF were compared between two groups using two-way repeated measures analysis of variance (ANOVA) with the Bonferroni post-hoc test. Differences were considered significant at values of P < 0.05. This statistical analysis was performed using a SPSS package (version 12.0, SPSS Inc., Chicago, IL).
Phenylephrine was used to preserve hypertension in SHR during acute ischemic stroke
Infarct volume, edema, and neurological deficits were significantly reduced
Cerebral blood flow inside the infarct area recovered faster in hypertensive rats
Cerebral blood flow outside the infarct area was higher in hypertensive rats
Sustaining preexisting hypertension may be valuable to acute stroke management
Acknowledgments
The authors are grateful to the excellent technical assistance of Xianfeng (Lisa) Zhang.
Sources of Funding This research was supported by the Intramural Research Program of NIH, NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams HP, Jr., del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J. Cereb. Blood Flow Metab. 1996;16:1236–1249. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- Amenta F, Di Tullio MA, Tomassoni D. Arterial hypertension and brain damage--evidence from animal models. Clin Exp Hypertens. 2003;25:359–380. doi: 10.1081/ceh-120023545. [DOI] [PubMed] [Google Scholar]
- Ayata C, Ropper AH. Ischaemic brain oedema. J. Clin. Neurosci. 2002;9:113–124. doi: 10.1054/jocn.2001.1031. [DOI] [PubMed] [Google Scholar]
- Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, Badger AM, White RF, McVey MJ, Legos JJ, Erhardt JA, Nelson AH, Ohlstein EH, Hunter AJ, Ward K, Smith BR, Adams JL, Parsons AA. SB 239063, a second-generation p38 mitogen-activated protein kinase inhibitor, reduces brain injury and neurological deficits in cerebral focal ischemia. J. Pharmacol. Exp. Ther. 2001;296:312–321. [PubMed] [Google Scholar]
- Bevan JA, Duckworth J, Laher I, Oriowo MA, McPherson GA, Bevan RD. Sympathetic control of cerebral arteries: specialization in receptor type, reserve, affinity, and distribution. FASEB J. 1987;1:193–198. doi: 10.1096/fasebj.1.3.2887477. [DOI] [PubMed] [Google Scholar]
- Brott T, Lu M, Kothari R, Fagan SC, Frankel M, Grotta JC, Broderick J, Kwiatkowski T, Lewandowski C, Haley EC, Marler JR, Tilley BC. Hypertension and its treatment in the NINDS rt-PA Stroke Trial. Stroke. 1998;29:1504–1509. doi: 10.1161/01.str.29.8.1504. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Dunn B, Todd JW, Warach S. Induced hypertension improves cerebral blood flow in acute ischemic stroke. Neurology. 2005;64:1979. doi: 10.1212/01.WNL.0000156360.70336.18. [DOI] [PubMed] [Google Scholar]
- Chileuitt L, Leber K, McCalden T, Weinstein PR. Induced hypertension during ischemia reduces infarct area after temporary middle cerebral artery occlusion in rats. Surg. Neurol. 1996;46:229–234. doi: 10.1016/0090-3019(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Chu X, Qi C, Zou L, Fu X. Intraluminal suture occlusion and ligation of the distal branch of internal carotid artery: an improved rat model of focal cerebral ischemia-reperfusion. J. Neurosci. Methods. 2008;168:1–7. doi: 10.1016/j.jneumeth.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Cole DJ, Drummond JC, Osborne TN, Matsumura J. Hypertension and hemodilution during cerebral ischemia reduce brain injury and edema. Am. J. Physiol. 1990a;259:H211–217. doi: 10.1152/ajpheart.1990.259.1.H211. [DOI] [PubMed] [Google Scholar]
- Cole DJ, Drummond JC, Ruta TS, Peckham NH. Hemodilution and hypertension effects on cerebral hemorrhage in cerebral ischemia in rats. Stroke. 1990b;21:1333–1339. doi: 10.1161/01.str.21.9.1333. [DOI] [PubMed] [Google Scholar]
- Cole DJ, Matsumura JS, Drummond JC, Schell RM. Focal cerebral ischemia in rats: effects of induced hypertension, during reperfusion, on CBF. J. Cereb. Blood Flow Metab. 1992;12:64–69. doi: 10.1038/jcbfm.1992.8. [DOI] [PubMed] [Google Scholar]
- Drummond JC, Oh YS, Cole DJ, Shapiro HM. Phenylephrine-induced hypertension reduces ischemia following middle cerebral artery occlusion in rats. Stroke. 1989;20:1538–1544. doi: 10.1161/01.str.20.11.1538. [DOI] [PubMed] [Google Scholar]
- Geeganage CM, Bath PM. Relationship between therapeutic changes in blood pressure and outcomes in acute stroke: a metaregression. Hypertension. 2009;54:775–781. doi: 10.1161/HYPERTENSIONAHA.109.133538. [DOI] [PubMed] [Google Scholar]
- Gerriets T, Stolz E, Walberer M, Müller C, Kluge A, Bachmann A, Fisher M, Kaps M, Bachmann G. Noninvasive quantification of brain edema and the space-occupying effect in rat stroke models using magnetic resonance imaging. Stroke. 2004;35:566–571. doi: 10.1161/01.STR.0000113692.38574.57. [DOI] [PubMed] [Google Scholar]
- Harms H, Wiegand F, Megow D, Prass K, Einhäupl KM, Dirnagl U. Acute treatment of hypertension increases infarct sizes in spontaneously hypertensive rats. Neuroreport. 2000;11:355–359. doi: 10.1097/00001756-200002070-00027. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Ito H, Yokoyama K, Makita K. Phenylephrine ameliorates cerebral cytotoxic edema and reduces cerebral infarction volume in a rat model of complete unilateral carotid artery occlusion with severe hypotension. Anesth. Analg. 2009;108:1631–1637. doi: 10.1213/ane.0b013e31819d94e3. [DOI] [PubMed] [Google Scholar]
- Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33:1315–1320. doi: 10.1161/01.str.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- Leoni RF, Paiva FF, Henning EC, Nascimento GC, Tannús A, de Araujo DB, Silva AC. Magnetic resonance imaging quantification of regional cerebral blood flow and cerebrovascular reactivity to carbon dioxide in normotensive and hypertensive rats. Neuroimage. 2011;58:75–81. doi: 10.1016/j.neuroimage.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lu H, Leoni R, Silva AC, Stein EA, Yang Y. High-field continuous arterial spin labeling with long labeling duration: reduced confounds from blood transit time and postlabeling delay. Magn. Reson. Med. 2010;64:1557–1566. doi: 10.1002/mrm.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Gallagher L, Gsell W, Graham D, Dominiczak AF, Macrae IM. Differences in the evolution of the ischemic penumbra in stroke-prone spontaneously hypertensive and Wistar-Kyoto rats. Stroke. 2009;40:3864–3868. doi: 10.1161/STROKEAHA.109.559021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Fisher M, Shen Q, Sotak CH, Duong TQ. Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann. Neurol. 2004;55:207–212. doi: 10.1002/ana.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rordorf G, Koroshetz WJ, Ezzeddine MA, Segal AZ, Buonanno FS. A pilot study of drug-induced hypertension for treatment of acute stroke. Neurology. 2001;56:1210–1213. doi: 10.1212/wnl.56.9.1210. [DOI] [PubMed] [Google Scholar]
- Schwinn DA, McIntyre RW, Reves JG. Isoflurane-induced vasodilation: role of the alpha-adrenergic nervous system. Anesth. Analg. 1990;71:451–459. doi: 10.1213/00000539-199011000-00001. [DOI] [PubMed] [Google Scholar]
- Shin HK, Nishimura M, Jones PB, Ay H, Boas DA, Moskowitz MA, Ayata C. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke. 2008;39:1548–1555. doi: 10.1161/STROKEAHA.107.499483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A, Zhang W, Williams D, Koretsky A. Multi-slice MRI of rat brain perfusion during amphetamine stimulation using arterial spin labeling. Magn. Reson. Med. 1995;33:209–214. doi: 10.1002/mrm.1910330210. [DOI] [PubMed] [Google Scholar]
- Smrcka M, Ogilvy CS, Crow RJ, Maynard KI, Kawamata T, Ames A., 3rd. Induced hypertension improves regional blood flow and protects against infarction during focal ischemia: time course of changes in blood flow measured by laser Doppler imaging. Neurosurgery. 1998;42:617–625. doi: 10.1097/00006123-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Sokrab TE, Johansson BB. Regional cerebral blood flow in acute hypertension induced by adrenaline, noradrenaline and phenylephrine in the conscious rat. Acta. Physiol. Scand. 1989;137:101–106. doi: 10.1111/j.1748-1716.1989.tb08725.x. [DOI] [PubMed] [Google Scholar]
- Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br. Med. J. 1973;1:507–510. doi: 10.1136/bmj.1.5852.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symon L, Branston NM, Strong AJ. Autoregulation in acute focal ischemia. An experimental study. Stroke. 1976;7:547–554. doi: 10.1161/01.str.7.6.547. [DOI] [PubMed] [Google Scholar]
- Waltz AG. Effect of blood pressure on blood flow in ischemic and in nonischemic cerebral cortex. The phenomena of autoregulation and luxury perfusion. Neurology. 1968;18:613–621. doi: 10.1212/wnl.18.7.613. [DOI] [PubMed] [Google Scholar]